| [打印本页][打印选项] |
| C.I. 冰染重氮组分5 (C.I. 37125) 生产工艺 CAS号 [97-52-9] 红色基B。 |
C.I. 冰染重氮组分5(C.I. 37125)生产工艺 CAS号[97-52-9]红色基B。
CAS名: Benzenamine, 2-methoxy-4-nitro-, 参考文献: Beil. 13, 390, E1, 121, E2, 194, E3, 888, E4, 903.
发明者: Soc.
Alsacienne Prod. Chim. 1897年。Winther, Laska, Zitscher 1911年。本色基盐酸盐CAS号 [71720-49-5]
用途: C.I.
酸性红38, 215, 226, 315, 405, 414。C.I. 酸性蓝300。C.I. 酸性绿12。C.I. 酸性黑31, 63, 107, 170。
C.I. 直接黑71, 122。C.I. 分散红41。C.I. 分散黑4。C.I. 溶剂红8, 13, 100, 122, 142。C.I. 溶剂紫1。C.I. 溶剂蓝53。
C.I. 溶剂黑27, 34, 35。C.I. 颜料黄74, 111, 198。C.I. 颜料红16, 19, 171。C.I.活性紫33。等。
生产工艺参考文献: 原版Colour
Index: BIOS 986, 278-285. FIAT
764 – Echtrot B Base 和 Echtrotsalz B neu. 未注明具体PB号。
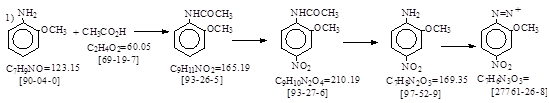
路线1: 醋酸或醋酐酰化法保护氨基。
BIOS 986,11-14(=胶卷PB 77764)No.2. Acet-o-anisidide (1-Methoxy-2-acetylaminobenzene) I.G.
Offenbach. 英国人译自德文,
Reaction equation: 略。Charge: 略。Yield: 略。Plant: 略。注:本文是英国人摘译自德文,但未说明译自哪个PB报告。
Process: 操作步骤: 中文摘译文,张澍声。《精细化工中间体工业生产技术》1996年。 P. 50.
日文摘译文。细田豊。《理论制造染料化学》1957年。P.654.
Raw materials:o-Anisidine (Orthsin No. 2304) 2600
kg. 这是西德奥芬巴赫厂的生产工艺。
Acetic acid, tech. pure No. 1309-1 1700
kg. (This may in part by replaced by recovered acetic acid)
Acetic anhydride No. 1310-1 50
kg.
For the first charge 1700 kg. acetic acid tech. pure is sucked into the acetylating pan
(item 1). For the following charges recovered acetic acid from previous charges
roused (400-500 kg. acetic acid 100%)
strengthened by the addition of acetic acid tech. pure to bring the total up to 1700 kg. tech. pure 2600 kg. of o-anisidine is then
charged.
Distillation I.
This reaction mixture is first boiled for 2 hr. under reflux and then with use of
dephlogmator the gradually diluted acetic acid is distilled off into the
storage vessel (item 3e); the cooling water meanwhile runs from the upper
run-off. The temperature falls first from 120
to 1160C. and then slowly begins to rise approx. 8 hr. after commencement of the
distillation. The acetic acid content of the aqueous distillate is at first 4% and increases continuously. When it
corresponds to 20% acetic acid,
which occurs when the temperature in the still reaches 160-1700C. the distillate is caught in the measuring
vessel (item 3d) and is used in the next charge.
The distillation is carried on until a still
temperature of 1980C. is
reached, after which at approx. 1800C, the cooling water to the dephlegmator is run off from the middle and later
from the bottom run-off. The end of the distillation is run without cooling
water.
Time of distillation I: approx. 36 hr.
Vacuum distillation I.
After shutting off steam, the pan is gradually
evacuated, during which the temperature falls from 198 to 1750C. At the end the vacuum should correspond to 72-73 cm. Hg. On slackening of the distillation,
which at first is very vigerous steam is again turned on and distillation
continued up to 1980C. Vacuum
and steam and steam are then taken off.
Time of vacuum distillation I: 7-8
hr.
Vacuum distillation II.
50
kg. acetic anhydride from
the measuring vessel (item 3d) is then added to the reaction mixture. Vacuum
and steam are again put on and the product distilled off up to 2000C. at 72-73 cm. Hg. Time of distillation approx. 3 hr.
The product is then discharged without
previous cooling into the cooling pans (Item No.2). Will cold it is broken up
and then ground in the disintegrator (item No. 3).
The final product shall not contain more
than 0.2% of unchanged o-anisidine.
FIAT 1313,I, 15. O-Acetaniside “Acetorthosin” I.G. Leverkusen 美国人译自德文。译者未说明译自哪个PB 报告。抄录如下。
Apparatus: 略。这是西德勒弗库森厂,即Bayer厂的生产工艺。
Materials: 1 – 900 kg o-anisidine. (邻氨基苯甲醚). 2 – 775
kg acetic anhydride.(醋酐 - (CH3CO)2 =102.09).
Procedure: 操作步骤: 无译文。这是Bayer 公司的生产工艺。醋酐的CAS号[108-24-7]. 文中(1)到(3)是设备号。
Add (1) to acetylator and over a period of 12 hrs. add (2) gradually. The
temperature soon goes to 60-700C. Run water through coil to maintain temperature. Heat slowly to 170-1800C. Acetic acid
distills over. It is about 95% strength.
Tests for completion of reaction: 100
grams after grinding and making acid with HCl, consumes not more than 0.1
gram NaNO2 . No color with R-Salt
after diazotizing.
Since the small excess of acetic anhydride distills off
slowly the product colors on too long heating at 1800C., 10 l. of water are added to the vessel and after
a short time of stirring, the last of the acetic acid is distilled off at 1800C. Cool to 1500C., blow to the flaking
machine feed tank and flake the product.
The recovery of 95-96% acetic acid is 88% of
theoretical. It is used for the acetylation of aniline or p-toluidine. The yirld of product is 98% of theory.
PB 70422, 1886-1888. Acet-ortho-aniside1ine. By Wollemann. 1943年6月23日。2页。 1.5美元。(德文工艺原件)未抄录。
PB 74022, 1889-1902. Nitro-ortho-anisidine
52, crude, wet. (“Echtrot” B base). 无日期,1.5美元 未抄录。
PB 70423, 2680. 3009-3014. Aceto-o-anisidine. 1936年和1937年 (德文生产工艺)未抄录。
路线1: 硝化和水解:
BIOS 986, 278-285. No. 154. 5-Nitro-o-anisidine Crude moist
(Fast Red B Base) 和 No. 155. Pure dry. I.G.Offenbach. 抄录如下。
以下是英国人译自德文,但译者未说明译自哪个PB报告?译文从反应式到投料量,略。注:译者理解不同,译文就不同。
Process: 这是西德奥芬巴赫厂的生产工艺。
Raw Materials:
Acet-o-anisidide 100% 500 kg. Sulphuric acid 600Be’ 940
kg. Nitric acid 100%, as approx. 400Be’ 210
kg.
Nitration:
710 l. water and 650
kg. sulphuric acid 600Be’. are mixed in the nitrator (item No.
1) and 210 kg. nitric acid 100% as 400Be’. added from the measuring vessel (item No. 4). The temperature of mixture is
brought to 250C. During 2 hrs. 500 kg. acet-o-anisidide 100% are
charged. Care must be taken at the beginning that the reaction commences.
Therefore 2 kg. sodium nitrite is
introduced at the beginning and thereafter regularly during the charging of the
acet-o-anisidide 7 kg. bisulphite liq.
38-400Be’ is dropped in. The smooth course of the reaction is to
be recognized by the gradual rise in temperature. Temperature control is
effected by rate of charging and by external cooling. When all the
acet-o-anisidie has been charged the temperature must have reached 400C. The mixture is
agitated for a further 1/2 hour. It is necessary to maintain the reaction temperature, if necessary,
by slight external heating, in order to obtain good results. The contents of
the nitrator are discharged through the hallow sharft to a nutsch (item 3) and
washed free from acid.
The moist nitro-body weighs approximately 950 kg. and has a solid content of
approximately 63%. The filtrate
collected in the vacuum receiver (item No, 4a) is blown to drain (抄注:to drain,
说明当时没有废水处理!)
Hydrolysis: 水解:
1650 l. water (or mother liquor from recrystallisation –
see process for 5-nitro-o-anisidine pure) and the product from 2 nitration batches, approx. 1900 kg. moist material, equivalent to 1000 kg. acet-o-anisidide, are charged
into the hydrolysis vessel (item No, 2) followed by 580 kg. sulphuric acid 600Be’. The mixture is heated to 950C. and agitated at this
temperature 13/4 hours. It is then cooled to 500C by the addition of ice. 170 kg. soda
ash is then added to neutralize part of the acid and the mass is diluted to
a volume of 8000 l. at a temperature
of 350C. by means of ice
and water.
The weakly basic 5-nitro-o-anisidine dissociates and precipitates from the solution which is still acid to Congo
Red (pH 1.8) whilst the more
strongly basic 4-nitro-o-anisidine remains
in solution. The 5-nito-o-anisidine is filtered on a nutsch and washed
acid-free with water. The filtrate and wash water are drawn into the pas (item
No. 4b) and the by-product is recovered according to the process for 4-nitro-o-anisidine. The crude 5-nitro-o-anisidine is used internally
as a moist intermediate. If crude 5-nitro-o-anisidine, dry, is required the material
is dried at 950C. in
vacuum dryer and then pump ground in a Simplex-Perplex mill provided with sieve
with 2 mm. slits.
For sale as Fast
Red B Base it must be recrystallised (see manufacturing process for 5-nitro-oanisidine pure – Fast Red B Base).
Method of Analysis: 略。 Plant: 略。以下是p. 282-285. 5-nitro-o-anisidine pure dry 即提纯,精制,摘录如下。
Raw materials:
5-nitro-o-anisidine crude moist, 100% 330.8 kg. Carboraffin 15
kg. Soda ash 2 kg.
Process: 精制:
11,500 litres of water or mother liquor, 15 kg. Carboraffin dry as approximately 33.1/3 %, “moist carbon” and 2 kg soda ash are charged into the
pressure boiler (item No, 1). 390 kg.
5-nitro-o-anisidine (act. wt.) – 330.8
kg. 100% is then charged. Steam is then blown on to the surface of the
material in the closed boiler until a pressure of 2 ats. is reached (temperature approx. 1200C.). This requires 11/2 – 2 hours. Agitation is continued a
further 20 minutes at 2 ats. and the
material is blown with steam through the Scheibler filter (item No. 2a) into
the cooling tank (item No. 3). After cooling to 300C. it is pumped through the filter press (item No.
2b). The press accommodates two
recrystallisation batches. The filtrate is used for the next recrystallisation in order to avoid loss of material on account of
its solubility in water. The excess
of mother liquor from condensate is utilized in the hydrolysis of acetyl compound (see process for 5-nitro-o-anisidine
crude moist.
The residues from the Scheibler filter are collected
and extracted with mother liquor in
the pressure boiler. The moist material is then dried in the
Venuleth-Ellenberger (这是一种耙式干燥器) dryer, heated with water at 950C. On discharge from the
dryer, the material passé through the “Express” sieving machine (item No, 4)
connected to it in order to remove or pulverize small lumps. The material is
then ready for dispatch.
Analytical method: (As for
5-nitro-o-anisidine crude moist)
Method for dye test for Fast Red B Base: 红色基B 染色测定:
The testing of the colour bases amounts to examination
of their physical condition (colour and finess), diazotisability, appearance of the diazo solution (colour
impurities etc., ) and shade.
Method of diazotizing Fast Red B Base: 重氮化测定: 这就是反应式中的重氮体,C7H6N3O3 = 180.14. 其CAS号[27761-26-8].
17 gm. Fast red B base is pasted with 30 cc. hot water and 7.5 gm.
Sodium nitrite added. After complete solution of the nitrite the paste is
cooled and added with continuous stirring in small portions to a mixture of 350 cc. cold water, 30 cc. Hydrochloric
acid 200Be’. The mixture is thrn allowed to stand 1/2 hour with frequent stirring. Filter
and add 15 gm. Sodium acetate dissolved
in about 40 cc. water. Adjust to the
required volume. Temperature of diazotization:
10 – 150C.
Preparation of the colour: 这就是织物上生成冰染红,实际上是合成C.I. 颜料红16.
(C.I. 12500) CAS号[6407-71-2]
Padding: Naphthol AS – BO [132-68-3] 3.5 gm. per litre.
Developing: Fast Red B Base [97-52-9] 1.7 gm. per litre. C.I. 颜料红生产工艺,请见PB
74037, 1763-1772. 1938年。
PB 25625, 463-469. I.G. Farbenindustrie A.G. Method
for producing 5-nitro-2-anisidine (T. E. A. Buero Report) 1937年5月。
共7页,1美元。本人未抄录。美国人介绍:This substance is identical with “Echtrot B-Base.” [97-52-9] It is produced from Aceto-o-
Anisidine [93-26-5] . A by-product is 4-nitro-2-anisidine.[99-59-2] 是C.I. 冰染重氮组分13,(C.I. 37130)。 Process and purification are
Described in
detail. In German. 其中CAS号和说明是本人加注。
PB 25625, 332-343. “Echtsalz B neu” 12页,1美元。1939年1月。美国人介绍如下。本人未抄录。
This is detailed laboratory
report on a new product and its properties. The product is obtained by diazotizing 4-nitro-o-anisidine („Echt
B-baa-c“) and combining with Naphthalene-1,5-disodium
sulfate. The New dye was found suitable for U. S. and for the tropics. In German. 这就是稳定重氮盐,C10H6O6S2.
2 C7H6N3O3 = 6446.56. 其CAS号[61925-55-1]. 国内有生产。
PB 25625, 344-346. Nitrosamine of 4-nitro-o-anisidine. 3页。1美元。1938年11月。 美国人介绍如下。本人未抄录。
Purified 4-nitro-o-anisidine
(=Echtrot B base) is diazotized, and rearranged by means of sodium hydroxide. The process is
described in detail. In German.
PB 25628, 3457. Geneal
Aniline works, INC. Fast Red salt B(I.G.)1美元。1937年7月。美国人介绍如下。未抄录。
Laboratory instruction are given for diazotizing 5-nitro-2-anisidine, for
the formation of the ZnCl2 double salt and for drying with a 50 –
50 mixture of Al2(SO4)3 . 6 H2O and MgSO4 anhydrous to
standarise the product to 20.5%. In English.
以上所述产品,其CAS号 [68540-82-9]. C7H6Cl3N3O3.
Zn = 350.85. 国内没有生产。
PB 82232, 693. “Azorotsalz B” 1946年 德文。2美元。美国人介绍如下。本人未抄录。
From 5-nitro-2-anisidine and 2-methylamino-5-sulfo-1-benzoic
acid. 本人未査CAS号。
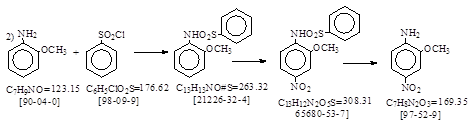
路线2: 用苯磺酰氯法保护氨基。
天津染料生产工艺汇编, 红色基 B 1980年。 P. 148-150 摘录如下。
操作方法: 缩合硝化:单批投(工业品)邻氨基苯甲醚440公斤,工业苯磺酰氯670公斤,液碱220公升,85%硝酸月83.6升,其余操作参照邻硝基对甲苯胺(即ADC 8)。 这是参照了BIOS
986, 301的生产工艺。
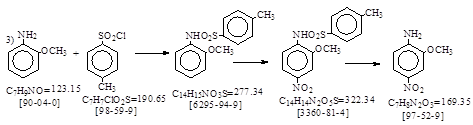
路线3: 用对甲苯磺酰氯法保护氨基。
上海染料生产工艺汇编,红色基B(2-甲氧基-4-硝基苯胺)1976年。 P. 207-208. 摘录如下。
操作方法: 1. 缩合: 这里是用对甲苯磺酰氯缩合。
缩合锅内放水1000升,升温至850C, 压入邻氨苯甲醚440公斤,然后将等分子比的对甲苯磺酰氯缓缓加入,并不断加入纯碱调整反应液pH = 8, 加料时间约1小时。测定终点:取反应物水溶液重氮化后与R盐在试管中反应呈橘黄色。终点到达后冷却至600C,用硫酸调整pH
= 6,再冷却至450C,放料过滤。收率99%。
2. 硝化: 硝化釜内放入530升氯苯,加入40公斤酰化物(干品重),调整温度至30-330C, 搅拌15分钟。然后加入1公斤亚硝酸钠,再逐渐加入82%硝酸19升,加料温度不超过500C, 再将410公斤酰化物(干品重)及82%硝酸74升,按比例加入,控制硝酸先加完10分钟,加料时间为3.5小时,反应温度控制在35-450C. 料加完后15分钟内升温至500C,保温1.5小时。反应完毕,冷却至300C过滤,滤饼用氯苯洗涤,然后再用清水洗至无酸性,出料,干燥,氯苯回收,干品熔点≥1690C. 收率85%。
3. 水解,中和: 水解釜内放入82-83%硫酸910公斤,调节温度到780C, 加入硝化物,加料时间为4小时,加料温度不超过900C,保温2.5小时。反应完毕,放入12吨水中稀释,稀释温度不超过500C, 同时用氨水中和,调整pH = 7.5. 于300C进行压滤,并用5%纯碱液洗涤,再用清水洗涤,吹干。干品熔点≥1370C. 收率95%。总收率: 79.9%.
加注: 这是文化大革命后的产物,原来是内部资料,但是后来有人抄录出书,而且不说明资料来源,变成他的了!
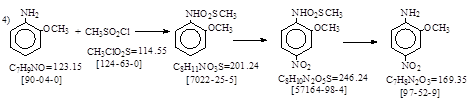
路线4: 用甲磺酰氯法保护氨基。
这是通过美国“化学文摘”CA
110: 175149. 找到的文献,Hoechst.
EP 292911 = JPS 63307848 = DE 3717833. 专利提到BIOS
986,但未提PB 报告,为什么?因为这是他们的内部资料,请注意:BIOS已出书,而PB报告是缩微胶卷,一般不会有人看。至少国内很多读者不知道。用苯磺酰氯保护氨基的问题,专利也是提到了BIOS
986.
国内外研究动态: 通过纸版美国化学文摘,抄录如下。
CA. 88:
104821. – Ser. Chim-Metal. 1977, 39(1), 63-7. (Rom)
CA. 92:
198053. – Chem. Ind (London) 1979,(23), 853-4.
CA. 132:
126971. – 上海环保科学。1999, 18 (10), 472-4. 废水处理。
CA. 134:
115496. – Green chem.. 2000, 2 (30).
104-5. 采用 HY Zeolite 提高酰化收率。
电子版美国化学文摘: 本人已无能为力。需要付费。
以上是草稿。 陈忠源 2016年12月8日。 供讨论用。
文章作者:陈忠源 |