| [打印本页][打印选项] |
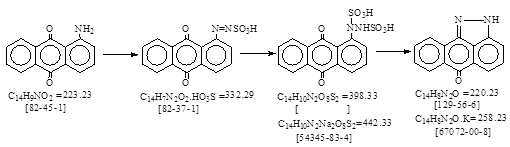
| CAS号 [129-56-6] 生产工艺 吡唑蒽酮 |
CAS号 [129-56-6] 生产工艺 吡唑蒽酮
CAS名: Anthra[1,9-cd]pyrazol-6(2H)-one, 参考文献: Beil. 24, E1, 276; E2, 108; E3/4, 713.
用途: C.I.还原红13, 34。 C.I.还原蓝25。C.I.还原黑8。C.I.颜料红195。(中间产品用途见国外文献。)
中间产品: CAS号[54345-83-4]. 名: 1,2-Hydrazinedisulfonic acid, 1-(9,10-dihydro-9,10-dioxo-1-anthracenyl)-,
disodium salt.
生产工艺文献:
BIOS 987, 128. (=胶卷PB 75860) Pyrazole anthrone 英国人译自德文。抄录如下。
反应式: 这是Mainkur厂生产工艺,译者未说明译自哪个德文原件?本人有加注。
Production 2000 kg. per year (100%)
To 760 l. 94% H2SO4,
is added 282 kg. nitrosyl sulphuric acid recognized at 100%. Actually 40% solution prepared at Hoechst is used.
466 kg. 1-aminoanthraquinone is stirred in cold and the mixture run at 15-170 for 4 hours, finally heating to 350C.
The diazo is poured on to ice, so that the temperature does not rise above 100,
requiring about 3200 kg. ice. Filtered by vacuum and washed with brine.
The diazo paste is then added to a solution prepared
from 580 kg. sodium bisulphite 100%
powder, 680 kg. NaOH 330Be’, 700 l. water, 600 kg. ice. It is stirred for a time at 17-200, heated to 700, salted out with 800
kg. salt, cooled, filtered and pressed.
The pressed hydrazine
paste is stirred into 2400 kg. monohydrate at 40-500, heated to 90-950, until the reaction is
complete, cooled to 750, and diluted below 750 with 2400
kg. water. The product is filtered
on a tile filter, washed and dried.
FIAT 1313,II, 160-161. (=胶卷PB 85172) Pyrazol anthrone. (Mainkur) 美国人译自德文。未说明资料来源。抄录如下。
Into a 1000 liter, homogeneously, lead-lined, jacketed,
agitated kettle are placed 735 kilograms of 660Be’ sulfuric acid (93%), 800 kilograms of nitrosyl
sulfuric acid (25%).
The two acids are mixed and with the temperature
adjusted to 150- 170 are added 240 kilograms 1-aminoanthraquinone. This addition is
made by hand through a 60-mesh screen to
remove lumps. After all 1-aminoanthraquinone has been added (about 2 hours),
the mixture is warmed slowly (1 hour) to 300-
350, and allowed to mix for 3-4 hours longer. The mixture is
then tested for completion of diazotization; a sample is diluted in cold water, and a clear solution should result and
excess of nitrous acid should be shown on starch-KI-paper. When diazotization
has been shown to be complete, the charge is blown over into 4000 liters of
water plus 2000 kilograms of ice in an acid-proof, brick-lined, 10,000 liter
tank.
This diazo slurry if filtered off on a porous stone
nutsch, 40 square meters, and washed with 230 Be’ sodium chloride
solution at room temperature; three 200 liter portions being used.
To the combined filtrate and first wash,, about 4000
liters, are added 100 kilograms of salt and 20 kilograms of zinc chloride. This precipitates the zinc salt of diazo compound and this
zinc salt is filtered off upon another nutsch and the nutsch cake washed with
230Be’ brine, and this additional diazo cake added to the principal
portion. It was stated that this additional diazo compound recovery amounted to
about 20 kilograms of zinc salt per
batch and that this was perfectly satisfactory for use. (抄注:请见C.I.冰染重氮盐36.)
The diazo paste obtained as above described is
converted as follows: A solution of sodium sulfite is prepared by adding 440
kilograms of sodium hydroxide solution 330Be’ (27%) and 700
kilograms sodium bisulfate solution 390Be’ (44%), to a 10,000 liter,
iron, agitated, jacketed tank, containing 4500 liters of water to which has
been added 500 kilograms of ice. To this solution the diazo paste id added at
170- 200 gradually in about 1 hour.
This slurry is agitated at 170- 200 for 15 hours, and this slurry must always remain alkaline. No control test is
run except test paper. The resultant slurry is then heated to 750 through the jacket in about 1 hour, and the entire mass must be in solution at
this point.
Then add activated charcoal, 8 kilograms, and filter through a clarifying
filter (either wood or iron press can be used), and wash the press with hot
water. The filtrate and washings, about 4000 liters, are then salted out with
400 kilograms of dry potassium chloride, and the slurry cooled to 200 and the hydrazine filtered off. The
press cakes from this press are then pressed in a hydraulic press to remove as much mother liquor as possible. The
cakes are wrapped in jute cloths in portions of about 3 cakes per cloth, and
pressed under 150 atmospheres (2250 lbs.) per square inch. The solids are
thereby increased from 25% to 50% and the cakes weigh about 600 kilograms after
pressing. (抄注:这里是1-蒽醌肼二磺酸二钾盐,二钠盐CAS号[54345-83-4])
These cakes are then dissolved in 2400 kilograms of
sulfuric acid 660Be’ at 400- 500, the cakes
being added in 5-6 hours in a homogeneously lead-lined kettle (3000 liters).
The temperature is then held at 400- 500 for 6-7 hours
until complete solution is obtained (Observe in test tube). The temperature is
then raised to 900- 950 and held for 1 hour, then cooled
back to 750 and an equal quantity of water is added (2400 liters)
while the temperature is held at 600 with jacket cooling. The
sulfuric acid should have a strength 400 Be’ at this point (from a filtered sample). If satisfactory, cool the
charge to 250 and filter on a porous stone nutsch and wash with cold
water until acid-free. Dry in vacuum at 1000 – 1100.
Yield = 204 kilograms, 85% of theory from 1-aminoanthraquinone. Melting
point = 2800 – 2820.
日文译文。细田豊。 《理论制造染料化学》1957年。P. 568-9. ヒラソ-ル アンスロン 译自FIAT 1313;BIOS 987. 抄录如下。
(1) 1-Diao-anthraquinone.
1 m3 ホモケ”ン铅张シ”ヤケツト釜に660Be’硫酸735
kgと,ニトロシル硫酸25% 800 kgを混合し,15-170て”1-アミノアントラキノン240 kgを60メツサユ金网を通して约2 hて”加え, 1 hて”30-350に温め3-4 h搅拌し,水4 t, 冰2 tに排出しシ”アソ”の沉淀をstone filterし,230Be’ NaCl水200
l.す”つて”3回洗う。滤洗液约4 tにNaCl 100 kg, ZnCl2 20 kgを加えてシ”アソ”のZn盐を沉淀させ滤過しNaCl水て”洗い约20 kgを回收し一绪に利用する。
(2) 1-Anthraquinonylhydrazine sulfonate.
NaOH 27%液440
kgに44% NaHSO3 700 kgを加えて造つたNa2SO3液と水4.5 t, 冰500 kgの混合液に前记シ”アソ”ヘ0-ストを17-200て”约1 hに加え15 h搅拌する。つき”に约1 hて”750に加热し,活性炭8 kgを加えて滤過し,滤洗液约4 m3にKCl 400 kgを加えて盐析し200て”滤過し,水压机にかけて50%固形约600 kgとする.
(3) Pyrazolanthrone.
660Be’硫酸2.4 t の中に前记cakeを40-500て”5-6 hに加えて完全に溶解するまて”6-7 h保温する。90-950に上け”1 h保温,600に冷して水2.4 tを加えて硫酸浓度を400Be’とし,250て”stone filterし,水洗,亁燥する。204 kg,收率85% /アミノ.
熔点280-2820.
PB 25625, 326-331. Echtrotsalz
AL 1934年3月8日。 1美元。有1-氨基蒽醌重氮化生产工艺。 请见C.I,冰染重氮组分36.
PB 25628, 3860-3867. Vojda and Max (General Aniline Works, Inc.,
(West Works), GRASSELLI, N.J. Pyrazole
anthrone. I.G.
Farbenindustries
A.G. TEA Buro-Report) Feb 1936- Apr 1939. 8 p. 售价 1美元。美国人介绍如下。
The method of preparation, preliminarly described,
consists of diazotization of 1-aminoanthraquinone, reduction to anthraquinonyl
hydrazine disulfonic acid, salting out with potassium chloride, splitting off the sulfonic group and
simultaneous ring closure in sulfuric acid. Legibility is not very good in
parts. In English and German. 今将部分原件抄录如下。
General Aniline Works, Inc. (West Works) Grasslli N.
J. Research Laboratory December 23, 1938.
Dr. A. M., Va jde Report No. 54. Pyrazole anthrone. 以下为实验室研究报告。
1. Introduction:
Pyrazole anthrone is a necessary intermediate for the
production of Indanthrene Rubine RD. Since
a prescription for the latter was desired, it was necessary first to study the
preparation of pyrazole anthrone. There are possible two ways.
I. From
1-chloroanthraquinone. II.
From 1-aminoanthraquione. 反应式:略!
The first method is sues in Germany as far as we known. However, because Hydrazine hydrate is
very expensive, and the supply of Pyridine is restricted, we have studied
the second way,
2. Discution of Experimentt:
The first step we investigated was the dehydration of
anthraquinonyl-1-hydrazine. The latter was made by diazotizing
1-aminoanthraquinone reduction of the diazo sulfate with sodium sulfite to the
hydrazine disulfonic acid, scission of the sulfonic groups with concentrated
HCl to anthraquinonyl-1-hydrazine hydrochloride, followed by decomposition with
sodium acetate or soda ash (DRP. 163447. Bayer, F., 8. 301).
Dehydration of the anthraquinone hydrazine by heating
in aniline and aniline hydrochloride, (DRP. 171293. Bayer. F.,8. 304) gave an
impure pyrazole anthrone, requiring recrystallization from nitrobenzene.
Heating with diluted HCl was without effect. The ring closure was easily
effected by heating in 96% sulfuric acid at 650C for 2 hrs. with
yields of 95% or better. However, further were in this direction was
discontinued. Since a sufficiently pure anthraquinonyl-hydrazine could be
obtained only in yield of about 70% by this way.
Attempts were made to eliminate the necessity of making
the free anthraquionyl hydrazine. It was thought that anthraqinonyl hydrazine
sulfate could be used for the ring closure. However, this could not be obtained
with constant composition, and ring closures on the press cakes gave pyrazole
anthrone for poor quality and only about 70% yield.
Since it was known that the sulfonic acid group of the
anthraquinonyl hydrazine disulfonic acid were easily split off by strong acid,
it was thought that a ring closure to pyrazole anthrone could be effected at
the same time, if concentrated sulfuric acid is used. This was found to be the
case. When the product obtained by salting out with KCl after the sulfite reduction of the diazo sulfate is dried and
heated in 10 parts 96% sulfuric acid at 850C for 1 hour, pyrazole
anthrone of good quality is obtained in a yield of about 75%.
The yield was thought to be too low. Ring closure was
known to give better yields: increasing the amount of KCl in salting out did not increase the yield. It was also
determined that diazotization proceeded to the extent of at least 96% by
converting the diazo sulfate to 1-hydroxyanthraquinone,
consequently,the losses seemed to occur during the sulfite reduction. It has
been found that when a 20% excess of
caustic soda beyond one equivalent, was added to the sodium bisulfate solution
used in the reduction, yields of pyrazole anthrone averaging around 90% were obtained.
There follows a preliminary prescription:
3. Preliminary prescriptin for pyrazole anthrone: 实验室合成方法:
(a)
Diazotizitation of 1-aminoanthraquinone. Dissolve 134 g of 1-aminoanthraquinone (tech.)
in 1072 g (584 cc) of 96% sulfuric
acid add at 100C 45 g sodium nitrite in small portions during approximately 15 minutes. Then charge 2155 g water at 150C. After
about 800 g water has been added during approximately 2 hours, a yellow
precipitate begins to form. At this point the remaining water may be added as
rapidly as the temperature of the mix can be maintained at 15-200C. Then stir for 1 hour, or until the product is
crystalline. The time up to this point is about 4 hours. Filter with suction
and press out thoroughly.
(b)
Anthraquinonyl-hydrazine disulfonic acid. Prepare first a
sulfite solution by adding 1190 g bisulfate
solution to a mixture of 415 g of
50% caustic soda solution and 625 g water.
Cool to 300C. The product from step (1) is sludged in 600 cc. water,
then add rapidly with good agitation, the above sulfite solution. Temperature
goes to about 350C, stir 1/2 hour, then raise
temperature to 500C in about 1/2 hour, hold
for 15 min., add 375 g KCl, allow to
stir while cooling to 250C. Filter. Press out and dry. Yield about 309 g (approx. 4% KCl)
(c) Pyrazole
anthrone. Dissolve the 309 g from step (b) in 3090
g of 96% sulfuric acid as rapidly as the heat to 850C for 1
hour, pour into 10 liter water, filter, wash free of acid and dry. Yield: 119 g (90% theory calc. on the
aminoanthraquinone used).
m. p. 281-2830C (Lit. 277-2780C). % N= 11.66 found. 12.72 calc. (11.88 for
product).
Report No. 54-a.
Pyrazole anthrone 1939年4月26日 实验室合成方法。抄录如下。
Addendum to Report No. 54. Dec. 23.
1938. In the report No. 54 of Dec. 23.
1938, the diazotization of 1-aminoanthraquinone was completed by charging water
very slowly into the sulfuric acid solution of the 1-aminoanthraquione and NaNO2.
In order to economize on the time necessary for the diazotization. It was
suggested to proceed in the reverse order, namely, to charge the sulfuric acid
mix into ice and water. This was done and it was found to give equality as good
results.
Diazotization of 1-aminoanthraquionone. 67 g
of a-aminoanthraquinone (tech.) was dissolved in 536 g of sulfuric acid 96% and
cooled to 100C, then 27.5 g NaNO2, finally powdered was
added during 15 minutes. After stirring for 1/2 hr. at
10-150C, the mixture was charged during 1/2 hr. into 540 g ice and 540 g water, while keeping the temperature below 150C.
After stirring for 1 hr., the product was filtered, pressed well, and worked up
to pyrazole anthrone as described in Report No. 54, using 1/2 the quantitative throughout.
Yield: 57 g (86.5% of theory). m.p.
279-2820C.
PB 73719, 2104-2110. Pyrazole anthrone. 1936年7月8日。未抄录。
产品分析方法: 德文。
10 g Pyrazolanthron werden mit 50 ccm Sprit + 300
ccm Wasser 15 Stunden verkugelt, mit 1200 ccm Wasser quantitativ in einen 3 L
kolben gespuelt und nach Zugatz von wiederen 200 ccm Sprit und 100 ccm
Natronlauge 400Be‘ 20‘ unter Rueckfluss zum Sieder erhitzt. Dann
wird heiss abgesaugt und mit heissem Wasser, das zunaechst noch etwas alkalisch
gehalten wird, bis zum voellig farblosen Ablauf gewaschen. Der Filterrueckstand
wird bei 1000 getrocknet und gewogen = alkaliumloesliche
Verunreinigung. Filtrat und Waschwasser wird noch heiss mit verduennter
Salzsaeure schwach kongosauer gemacht, und nach voelligem Erkalten das
abgeschliedene Pyrazianthron abgesaugt und eben kongoneutral gewaschen. Trocken
bei 1000 = reines Pyrazolanthron.
Es soll 95%ig sein. Trockengehalt: 99-100%. Aschegehalt: nicht ueber 1%.
产品分析方法: 英文译文。抄自FIAT 1313,I, 526. (=胶卷PB
85172)。但译者未说明资料来源?
Ball mill a 10.0 g. Sample with 50 cc., Ethanol and 300 cc. Water for 15
hours. Rinse out the mill into a 3 l. flask with 1200 cc. Water. Add 200 cc.
Ethanol and 100 cc. Sodium Hydroxide solution, 400Be’. Heat to
reflux temperature and hold 20 minutes. Filter hot and wash with hot water
containing a trace of Sodium Hydroxide until the washings are colorless. The
filtrate and washings are precipitated with Hydrochloric acid to a weak Congo
acidity, cooled and filtered. The precipitate is washed with water until
acid-free and dried at 1000C. The weight is reported as the assay
figure. It should be 95% or over for acceptable material. The ash is also
determined: it should not becover 1%.
国内生产工艺:
天津染料生产工艺汇编。 吡唑蒽酮。1980年。P. 223. 抄录如下。
A. 亚硝酰硫酸制备: 将硫酸850公斤放入重氮罐内夹套降温100C以下,加入亚硝酸钠63公斤,控制温度20-400C, 加完升温至700C, 搅拌30分钟,控制温度70-750C.
B. 重氮化: 将重氮罐内物料降温至150C以下,加入1-氨基蒽醌温度控制400C以下,加完料搅拌3小时,温度控制40-500C.
在稀释罐中加入碎冰1300公斤,将重氮液慢慢加入,控制温度150C以下,过滤,滤饼为重氮盐。
C. 还原: 在还原罐内加入水2000升,亚硫酸氢钠214公斤和苛性钠160公斤,搅拌30分钟,夹套冷却至100C以下,加入重氮液滤饼,温度控制在150C以下,pH
= 9-10,搅拌30分钟,升温至17-200C,保温10小时,升温至650C,在65-700C保温10分钟并加入工业盐600公斤,搅拌1小时,静置2小时,降温至300C压滤,滤饼为肼盐折干品320公斤。
D. 闭环: 将硫酸1800升,放入闭环罐内放入肼盐滤饼,温度控制在40-450C,保温3小时,酸度为81-84%,升温900C,在90-950C保温1小时,降温600C以下,将水细流注入罐内温度不超过600C,酸度控制为45-49%,降温至300C,压淋干燥得步骤蒽酮106.2公斤。
国外研究动态:
捷克专利。 CS. 201997. 1980年12月31日。 主要是合成1-蒽醌肼二磺酸钠盐。
美国专利。 US. 5385842. 1995年1月31日。主要是1-蒽醌肼二磺酸钠盐的应用。
加注:
译文和引用文都应该写上资料来源,这是技术人员应该遵守的,最基本的职业道德。美,英的上述译文不说了。然而,当今仍有一些出版物有这类问题出现?难道你的翻译没有错?编写中也没有抄错?
陈忠源 2017年2月28日 于 无锡 明辉国际。
文章作者:陈忠源 |