| [打印本页][打印选项] |
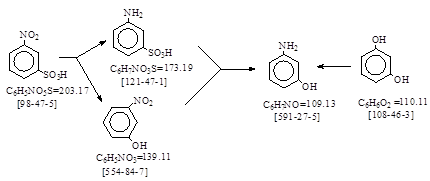
| CAS号 [591-27-5] 生产工艺 间氨基苯酚 |
CAS号 [591-27-5] 生产工艺 间氨基苯酚
CAS名: Phenol, 3-amino- 历史参考文献: Beil. 13,401; E1,128; E2,209; E3,931; E4,952.
用途: 医药。染料:毛皮黄,毛皮棕。直接黑155, 168。有机合成。反应类别: 磺酸基水解,硝基还原。羟基氨化。
生产工艺参考文献: 按本人手头资料整理如下。
BIOS 986, 400-401.(=胶卷 PB 77764) No. 201. m-Aminophenol.(I.G. Ludwigshafen)。英国人译自德文。抄录如下。
反应式: 本人有加注,译者未说明资料来源,这是老工艺。(有三条工艺路线)。
间氨基苯磺酸钠:C6H7NO3S.Na=195.18. CAS号[1126-34-7]. 间氨基苯酚钠:C6H7NO.Na=131.13. CAS号[38171-54-9]
Metanilic acid is dissolved in caustic soda and heated
with caustic potash, first to 240-2450C and finally to 2600C;
the melt is diluted with water, the sodium bisulphite filtered off hot and the
crude m-aminophenol precipitated with hydrochloric acid, filtered and washed.
The crude product is distilled at 1800C / 6 mm.
The materials usage per 1,000 kg. crude m-aminophenol
are:- 1,930 kg. metanilic acid. 475 kg. caustic soda 100%.
2,040 kg. caustic
potash. 3,140 kg. hydrochloric acid 30%.
The usage of crude meta-aminophenol per 1,000 kg. of
pure m-aminophenol is 1,290 kg
Yield of crude m-aminophenol – 82.2% theory from metanilic acid. Yield of pure m-aminophenol – 77.6% theory from crude m-aminophenol.
The average monthly production in tones from 1938 to 1944 inclusive of pure
m-aminophenol was nil, 0.5, 0.5, 0.7, 0.6, nil, nil.
BIOS 1153, 251-253.(=胶卷 PB 85687)m-Aminophenol.(Ludwigshafen). (Crude and distilled) 英国人译自德文。抄录如下。
Scale: 4.16 kg. mol. Materials: Mol. Ratio. Materials: Mol. Ratio.
Metanilic acid (75% acid) as 100% (M.W. 173) 720 kg.1.0. Caustic soda liquor
34.5% 510 kg. 1.06
Caustic potash 760 kg. 3.26. Hydrochloric acid 30% 1100 kg. 2.16.
Procedure: A. Fusion:
The metanilic acid (as free acid 75% st.) (= 720 kg.
100%) is charged into 510 kg. of caustic liquor and the solution tested for
alkalinity to phenol phthalein. The solution is dropped in two parts into a
heated blow-egg and then blown at 60-700 into the melt vessel
containing 760 kg. of caustic potash and 50 l. of water heated to 240-2450C. The whole
quantity of metanilate solution is blown over a period of 5-6 hours with the
temperature in the melt vessel maintained at 240-2450C.
The temperature is raised to 2600 and held for 2 hours. The mass is only stirred with
difficulty. It is then diluted with 150 l. of hot water and the thinned melt
run into the melt-solution tank containing 750 l. of water.
The crystalline sodium sulphite is filtered hot on a
nutsch and washed three times with 150 l. each of hot water. The 1st wash goes forward with the main filtrate and the 2nd and 3rd wash are returned as drawn-out water for the next batch.
The filtrate is blown to the precipitating tank and the
m-aminophenol precipitated by addition of 30% hydrochloric acid until only just
alkaline to Brilliant Yellow (1100 kg. normally required). It is stirred until
cold, dropped to a nutsche and washed with cold water. The mother liquor and
wash are discarded.
The crude m-aminophenol is charged back into the same
vessel and melted up with 450 l. of hot water, stirred until cold and
crystalline, filtered on the nutsche, and the liquor blown to drain. The
product is washed with 200 l. of cold water, and the wash returned as
melting-up water for the next batch.
The paste is melted up in a melting cylinder, separated
from the water and rub into a cooling pan where it is allowed to set.
Yield =
375-380 kg. crude. B. Distillation:
The combined product from 4 batches is distilled in
vacuo. A small pre-running over followed by the main product at 1800C. at 6 mm. The fore-
and end-runnings are returned to the next distillation.
4 x (375-380) kg. crude à 1175 kg. distilled. Theory yield 100 parts metanilic acid à 63 parts m-aminophenol.
Works yield: 100 parts à 52-53 parts crude. 100 parts crude à 78 parts distilled. 100 parts metanilic acid à 40.8 parts dist.
Yield - 64.8% of theory. Quality: C.Pt. not less than 1200 (pure 120.920).
Plaant: The main plant items are: Melt pan: 1.8 m3 with Gitterruehrer, 36 r.p.m., and breakers; gas heated.
Stll: Agitated still with
Balkenruehrer, 38 r.p.m. 1.5 kw. Motor. Gas heated.
细田豊《理论制造染料化学》1957年。P. 459. m-Aminophenol. 译自PB 85687. 抄录如下。
メタニル酸720 kg(100%)を75%ヘ0-ストの形て”NaOH 34.5% 510 kgと60-700て”溶し, KOH 760 kg + H2O
50 lに240-2450て”5-6 hかかつて加え,2600に2 h搅拌後水150 lを加え水750 lに排出, Na2SO3を滤過し湯150 lて”3回洗い, 後の2回は次回の敷水とする。滤液を盐酸约1.1 tてフ”リリアントエロ-纸にアルカリ性にし冷して滤洗する。粗m-アミノフエノ-ルを湯450 lと搅拌しなか”ら冷して滤過し,水200 lて”洗い洗液は熔融物をうすめるのに用いる。ヘ0-ストを溶かして水と分ける。粗制375-380 kg。4回分を集めて6 mm 1800て”蒸馏する。粗制の78%。收率64.8%。
张澍声 编译。《精细化工中间体工业生产技术》1996年。P. 88-89. 间氨基苯酚。 译自BIOS 1153,251. 抄录如下。
(一)碱熔: 将720 kg 100% 间氨基苯磺酸510 kg 34.5% NaOH溶液中,检验对酚酞为碱性。溶液在60-700C压入1800 L碱熔锅中,锅内1装有760 kg 100% KOH和50 L水,并加热到240-2450C.整个间氨基苯磺酸钠溶液于5-6小时压入,碱熔锅的温度保持在240-2450C。
加完后温度上升至2600C,保持2小时。物料搅拌有困难。然后用150 L热水稀释,稀的熔融物再流入装有750 L水的溶解锅。趁热抽滤出亚硫酸钠结晶,水洗3次,每次用150 L热水,第一次洗水与滤液合并,后两次洗水用于下次反应。
滤液压往沉淀槽,加入1100 kg 30% 盐酸使对亮黄试纸刚好为碱性,间氨基苯酚沉淀出来。搅拌冷却,抽滤,冷水洗涤,母液和洗水弃去。粗间氨基苯酚再用450 L热水熔融,搅拌冷却并结晶,抽滤。滤液弃去,产品再用200 L冷水洗涤,洗水用于下批熔融。
滤饼再熔融,分离出水,放冷,得到375-380
kg 粗品间氨基苯酚。
(二)蒸馏: 将4批产品合并,真空蒸馏。少量前馏份过去后,随后在1800C, 6 mmHg 得到主馏份。前馏份和尾馏份回到下次蒸馏中。
每100份粗品间氨基苯酚得到78份精制品。收率64.8%,熔点1200C, 纯品120.920C。
PB 17692, 705. m-Aminophenol. 未抄录。
PB 25602, 1107-1109. No. 2201-1. m-Aminophenol. 德文生产工艺。未抄录。
PB 70063, 330-333. Manufacture of 3-aminophenol (Fuscamin
G) By Schweizer. 1945年10月17日德文生产工艺。未抄录。
PB 70189, 6628. 德文间氨基苯酚产品分析方法。(Nr.
248) 未抄录。
上海市有机化学工业公司。《染料生产工艺汇编》 1976年。P. 40-41. 间氨基苯酚。抄录如下。
1. 碱熔: 收率74%。碱熔锅内置固碱或液碱折100% 945公斤。以加热融化或蒸浓至2750C,逐步加入间氨基苯磺酸钠盐溶液(折100% 间氨基苯磺酸945公斤)。加完,继续保持2750C反应45分钟。稀释于亚硫酸钠洗液中,加水调整到3800升。分离除去亚硫酸钠。
2. 酸化及蒸馏: 收率81%。分离去亚硫酸钠后的间氨基苯酚钠溶液2800升,在500C以下加入31% 盐酸至中性。降温到350C,析出粗氨基苯酚。过滤后,将滤饼加入蒸馏锅,在190-2500C
/ 700-740毫米汞柱,馏出精制间氨基苯酚。经制片机得片状品。总收率60%。抄注: 本资料已在:章思规 主编《精细有机化学品技术手册》科学出版社出版,1991年。P. 124 出版。
国内研究动态:
间氨基苯酚用CO2酸化简报。[J] 化学工业技术资料(染料及中间体专业分册)1965, 4, 41. 摘录如下。
南京化工厂用CO2代替盐酸酸化进行试验,并得到良好的结果。以下,略!
刘江琼 彭孝军。(大工)间氨基苯酚合成工艺研究进展。第六届染料学术报告会《论文集》 1997年9月。P. 190-191.
本文介绍几种合成工艺路线,结论是用间苯二酚氨解法为最佳,无参考文献。
刘江琼 彭孝军(大工)。间氨基苯酚及间苯二酚合成工艺研究进展。[J] 染料工业,
1998, 1, 19-23. 摘录如下。
本文重点:2.2. 间苯二酚氨解法。2.2.1. 气相法氨解。2.2.2. 液相氨解法。无具体合成工艺, 引用文献9篇。
参考文献: 共24篇。
梁 诚 (南京化工厂)。间氨基苯酚生产与发展。[J]染料工业,
2001, 1, 14-15(23). 请见原刊物。
董安周 李广学 等 (安徽理工)。间苯二酚氨解法合成间氨基苯酚工艺研究。[J] 染料与染色,
2015, 1, 39-41. 摘录如下。
实验方法: 在1 L带搅拌器高压釜内加入间苯二酚100 g,25% 氨水400 g,四水合七钼酸铵20 g,用氮气置换釜内空气3次,充压0.5 MPa后升温至2100C,在压力2 MPa下反应6小时。结束,降温,过滤出的滤渣可以回收使用,用无水乙醇洗涤滤饼3次,经过滤,分馏,蒸馏得到粗品间氨基苯酚,再用正丁醇进行重结晶提纯,得到纯度很高的间氨基苯酚。
产物用LC-20AT型高效液相色谱仪进行含量测定,计算产物收率。HPLC检测色谱条件:检测波长274 nm;固定相C18;柱温250C;流动相V(甲醇)/ V (水)= 20/80.
结果与讨论: 略。参考文献: 12篇。
董安周 胡小燕 等(安徽理工)。间氨基苯酚制备方法及Bucherer反应进展。[J]染料与染色, 2016, 1, 43-47. 摘录如下。
本文介绍几种制备方法,未见实例。参考文献: 22篇。
与作者讨论: 关于Bucherer
reaction. –NH2 à -OH.
Bucherer reaction: The conversion of a naphthylamine into the corresponding naphthol in the presence of sulphite or bisulphite ions in aqueous solution at high temperatures.
The reaction gives almost quantitative yields (in either direction) with substituted
naphthalene derivatives and with resorcinol but not with derivatives of benzene and other aromatic compounds.
如何理解文中的间苯二酚Bucherer催化氨解法。
国内申请的有关专利: CN 10187766. CN 101362710 和 CN 103804242等。
加注: 希望大家共同讨论!
陈忠源 2017年8月13日星期日 于 无锡 明辉国际。
文章作者:陈忠源 |