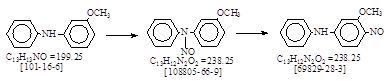| [打印本页][打印选项] |
| CAS号 [69829-28-3] 生产工艺。 3-甲氧基-4-亚硝基二苯胺。 |
CAS号 [69829-28-3] 生产工艺。 3-甲氧基-4-亚硝基二苯胺。
CAS名:Benzenamine, 3-methoxy-nitroso-N-phenyl- 历史参考文献:Beil. 待检索!
用途:冰染重氮组分47。 分数染料(见US 4239894.)。电子化工产品等。 LookChem网无登录。
BIOS 986,
400. (=胶卷PB 77764)。 3-Methoxy-4-nitrosodiphenylamine. (I.G. Hoechst.)。 英国人摘录,只有反应式。
反应式: 本人有加注,暂未找到德文原件。
Plant: 设备:略。(编号:(1) - (20))。
Process: 生产工艺: a)
Nitrosation : a) 亚硝化:
180 kg.
3-methoxydiphenylamine (at 100%) are dissolved in 350 l. (= 522 kg.) of
chloroform in (1). 60 l. of 600Be’ surphuric acid are run in from (3) and 150
l. of water then added. The temperature
is adjusted to 200 and 125 l. of sodium nitrite solution (40% strength) then
run in from (2) during 2 hours. After
standing for 24 hours the chloroform solution is separated from the aqueous
acid in (5) and discharged into (6)
b) Rearrangement: b) 重排:
50 kg. of
methanol are added and 40 kg. of hydrogen chloride gas passed in at 5-100 from
(7) in the course of 5 hours. The
hydrogen chloride is produced from 116 l. of 30% hydrochloric acid (from (8)
and 185 l. of monohydrate from (9). The
thick paste of the hydrochloride of the nitroso-compound is stirred for 11/2
hours, diluted with 80 l. of chloroform from (4) and discharged into (10).
c) conversion to base: c) 转换成色基:
(10) is
charged with about 4000 l. of water, 380 l. of caustic liquor (35%) recovered
from the nd extraction of the preceding batch, and 1000 kg. of ice. The addition of the nitroso hydrochloride
must be done with a temperature below 10℃.
After vigorous stirring it is allowed to settle in (12) for 24 hours and
the aqueous solution of nitroso run through (13) to (15). The chloroform is returned to (10) where it
is again stirred with 4000 l. of water and 380 l. of fresh caustic liquor. It is again separated in (12), the chloroform
being run to (14) and the caustic liquor returned to (10) for the extraction of
the next batch.
To the first
extraction liquor, 15 kg. of Kieselguhr are added and any remaining chloroform
removed by blowing air through for 5 hours.
The solution is then filtered through(16) into (17).
张澍声 《精细化工中间体工业生产技术》 1996年。P. 26-27.
3-甲氧基-4-亚硝基二苯胺。译自BIOS 1149, 70.
(1)亚硝化: 180 kg 100% 3-甲氧基二苯胺溶解于350 L氯仿,流入60 L 78% 硫酸和150 L水。温度调节到20℃,于2小时内流入125 L 40% 亚硝酸钠溶液,放置24小时后,分离出氯仿溶液。
(2)重排: 向氯仿溶液中加入50 kg甲醇,于5小时内在5-10℃通入40 kg氯化氢气体,氯化氢是由116 L 30%盐酸和185 L硫酸一水合物生成的。稠厚的亚硝基化合物盐酸盐浆状物搅拌1.5小时,用氯仿·稀释。
(3)转换成色基: 在锅中加入4000 L水,380 L 33% 氢氧化钠溶液(由前次反应第二次萃取回收的)的1000 kg冰,亚硝基物盐酸盐的加入必须在10℃以下进行。强烈搅拌后静置24小时,分离出亚硝基物的水溶液,氯仿回到锅中,再与4000 L水和380 L新的氢氧化钠溶液搅拌,再分离,氯仿收集后蒸馏,氢氧化钠溶液用于下次反应的第一次萃取。
向第一次萃取液中加入15 kg 硅藻土,通空气5小时除去所有残留的氯仿,溶液然后过滤,滤饼供还原。
FIAT
1313,III, 144. Laboratory Process. 4-Nitroso-3-methoxy-diphenylamine. 译自德文(无资料来源)。
404 g. (equals
2 mols) of 3-methoxy-diphenylamine of 96.1% purity (equals 388 g. pure) is
dissolved in 1700 cc. of warm pyridine-free denatured alcohol and is treated
slowly with 600 cc. chemically pure hydrochloric acid. The mixture is cooled with good agitation to
0-5℃. whereupon
there precipitates the finely crystalline hydrochloride of
3-methoxy-diphenylamine. To this slurry
is added slowly in drops a solution of 140 g. sodium nitrite in 200 cc. of
water at 0-5℃. After all nitrite has been dropped in, the
temperature is raised to 20℃. and is held there for one hour.
The reaction is cooled again to 0-5℃. and into the red-brown solution (from which
there already will have precipitated some dark red crystals) is led 200 g. of
hydrochloric acid gas. The temperature
must not rise above 10℃. After the gassing, the
temperature again is raised to 20℃., and held there one hour. Then the reaction is cooled as low as
possible (-5℃.) and is
filtered to isolate the dark red crystalline mass of the hydrochloride of 4-nitroso-3-methoxy-diphenylamine,
which is washed with 200 cc. of saturated salt solution.
国内研究动态:
大连工学院。 凡拉明蓝FG色基之试制。 [J] 有机化学工业技术报导, 1958, 11, 60-61.
试验是按照BIOS 1149 及 FIAT 1313第三卷的资料复制,所以这里不再抄录。
国内专利动态:
曹维孝 曹维伟 冯新德 (北京大学)。 中国专利 CN 1034711 – 1989年8月16日。 取代二苯胺-4-重氮盐的制备方法。
二苯胺重氮盐与甲醛缩合制得的重氮盐树脂,是制备负性预涂感光印刷版(商业上称负性PS版)的感光涂层原料。…. 至今在文献中未见有关3-甲氧基二苯胺-4-重氮盐的制备方法(这是原文!)。以下是本人抄录的专利原文:
实例3. 3-甲氧基-4-亚硝基-二苯胺的制备:
于100毫升三口瓶中加入2.5克(12.6 毫摩尔)3-甲氧基二苯胺,50毫升95% 乙醇,用冰冷却至 -5℃,于其中加入4毫升浓盐酸,在搅拌下,将1.06克(16.4毫摩尔)亚硝酸钠溶于2毫升水中的溶液,于0℃ 以下,慢慢滴入以上溶液中,滴完后继续搅拌反应10分钟,将之倒入冰水中,用10% 氢氧化钠水溶液将混合液的pH 调至7,各用50毫升氯仿提取两次,合并提取液,在室温减压下,于回旋蒸发器中除去溶剂,残留物为N-亚硝基-3-甲氧基二苯胺,(本人抄注:[108805-66-9]),重2.4克(产率85%)粘稠油状物,将改产品溶于3毫升甲苯中,于25℃把此溶液滴入盛2.4克氯化氢甲醇溶液(32%)的三口瓶中,滴完后继续反应1小时,于反应液中加10% 氢氧化钠溶液将pH 调至12,再搅拌30分钟。分出水层,于其中慢慢滴加30%的硫酸,至有多量结晶析出,过滤,得深蓝色有金属光泽的3-甲氧基-4-亚硝基二苯胺2.4克(84%)。熔点153-154℃。
元素分析:计算值(%)C
67.73, H 5.20, N
11.93
实验值(%)C
68.40,H 5.30,
N 12.30.
学习与思考:
学无止境,如何编写产品索引,便于国内同行的利用,少浪费的有用的时间,少做点重复的工作,是本人终身的奋斗目标,至少应该了解点产品的发展历史,
现在提出大众创新,我想也不影响去了解历史,了解过去,特别是有些技术是买不来的,但问题是:因为二战得到的免费资料至今无人好好利用,我想上述专利应该是一个例子(当然这是个别的例子。)。如果国内大学生受学校条件限制,哪么出国留学的学生,对此,未知有多少人知道这类资料!
如果说,北京大学没有本人所说的特种文献,那么北京中国科学院就有进口的全套特种文献资料,这就是本人至今一直在说的资料。上网已超过2年,1945年以前公开的资料,国内有进口,为什么还会出现上述问题,唯一的答案是:上述历史资料至今无人编目,本人利用美国人的CAS号上网,这只是开始!
工欲善其事必先利其器:
本人所理解的器,应该是想尽办法多了解一些已经公开的历史文献,这些是详细,具体的生产工艺文献!特别是德文原件,因为本人发现美国人或者英国人的译文不完全,有的有点小错(已在以前上网的资料中提到过!)。
陈忠源 2018年6月18日星期一。
文章作者:陈忠源 |