| [打印本页][打印选项] |
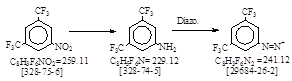
| C.I. 冰染重氮组分 16 (C.I. 37045) 生产工艺。 CAS号 [328-74-5] |
C.I. 冰染重氮组分 16 (C.I. 37045) 生产工艺。 CAS号 [328-74-5]
CAS名:色基: Benzenamine, 3,5-bis(trifluoromethyl)- 历史参考文献:Beil. 12. E3, 2498.
重氮体:Benzenediazonium,
3,5-bis(trifluoromethyl)-, CAS号 [29684-26-2].
发明者:Müller 1932年。 商品名:Echtorange GGD Base. (= Fast Orange GGD base. = [328-74-5])。
用途:冰染色素,除草剂。医药等。LookChem网登录生产与经营单位93家。
反应式:本人有加注。CAS号 [328-75-6] 已上网。
BIOS 986, 417.(=胶卷PB 77764)。 3,5-Di-(trifluoromethyl) aniline (Fast Orange GGD
base). I.G. Hoechst.
英国人摘录:Written
process was not available, but the “route” is 此页只有反应式:
BIOS 1149, 8.(=胶卷PB 80376)。 Fast Orange GGD Base. (3,5-di(trifluoromethyl)aniline). 英国人译自德文(无资料来源)。
Plant: 1. Iron reducer, 4.5 cu.m., with stirrer,
heating jacket and separating device.
2. Iron measure vessel, 500 l.
3. Filter press.
Materials: 1-Nitro-3,5-di(trifluoromethyl)benzene. B.p. 185-1900. Sp. Gr. 1.55. Iron turnings. Acetic acid.
Process:
The reducer
is charged with 500 kg of iron turnings, 500 l. of water and 20 kg of acetic
acid, and heated to the boil. During 32
hours, the nitro-compound (600 kg) is run in a thin stream. After completion the batch is boiled for a
further 6 hours, allowed to settle, and the oil blown off by means of a
separating device through a press.
Yield = 505
kg = 95% theory. Quality: B.Pt. 1890. C.Pt. + 3℃. Sp. Gr.
1.48.
Usages per tone
of product:
1-Nitro-3,5-di(trifluoromethyl) benzene
1190 kg. Iron borings 1000 kg.
Acetic acid 40 kg.
Electricity 500 kw.h.
Steam 15 tones.
Water
600 cu.m. Air
900 cu.m.
FIAT 1313,I, 53-54.(=胶卷PB 85172)。 5-Amino-1,3-xylene hexafluoride. I.G. Hoechst. Fast Orange GGD Base. 美国人译自德文。
Apparatus: a) 800 l. tile-lined reducer vessel fitted
with a ship’s propeller type agitator, 120 RPM.
b) 3 cbm iron vessel with an anchor
type agitator, 50 RPM, fitted for steam distillation.
Charge: 44 kg water. 155 kg iron powder. 4 kg hydrochloric acid, crude. 195 kg nitro Echtorange GGD, mol 259.
Process: 操作步骤: 美国人也未说明资料来源,为什么?
The water,
iron powder and hydrochloric acid are added to the 800 l. reducer under
agitation and heated to 100℃. At 100-102℃. 195 kg of nitro Echtorange GGD
(5-nitro-1,3bistrifluoromethylbenzene) are permitted to run into the reducer
slowly, i.e., as the reaction permits.
This requires about 8-10 hours.
After all the nitro Echtorange GGD has been added, the charge is boiled
for an additional 4 hours using a reflux condenser; then sampled and tested to
determine whether reduction is complete.
Upon completion of the reduction the charge is run to the 3 cbm iron
vessel and the reducer washed out several times with hot water. The Echtorange GGD base is then distilled off
with steam and well separated.
The material
distills over with water in the proportions of about 1:5. First a small portion, which is not
completely soluble in hydrochloric acid, distills over as foreshot. This, determined by frequently taking of test
samples, is drained off. Towards the end
of the steam distillation the bse comes over slightly yellow colored. This portion is collected as second runnings
and with the foreshot purified by fractional distillation at a temperature of
about 185-190℃. under
normal pressure. About 90% of the
material obtained from portion is GGD.
The water
separated from the steam distillation mixture still possesses the extraordinary
unpleasant odor of the base. This water
can be reused in the next charge for washing out the reducer by transferring it
directly from separator.
In order to
remove any traces of water which may still adhere to the base after separation,
the material including suitable additional charges is heated in a kettle for
some time at 100℃. until it is
completely dry, and then run thru a pressure filter into 200 l. tinned-iron
shipping drums.
Yield: 195 kg
nitro Echtorange GGD à 154 kg Echtorange GGD base. M.W. 299. (抄注: 分子量应该是229. 说明FIAT 也有错!)。
Another
process operated on a larger scale is as follows: (抄注:下面方法与BIOS 1149相同。)。
1) 1 – 4.5 cbm steam jacketed iron reducing
kettle, with iron agitator, steam inlet pipe and separating device.
2) 1 – 500 l. measuring vessel. 3) 1
– filter press.
Charge: 600 kg Nitro Fast Orange GGD, B.P. 185-190℃.
sp.gr. 1.55. 500 kg iron
turnings. 20 kg acetic acid.
Process:
500 kg of
iron turnings, 500 l. of water and 20 kg of acetic acid are charged into the
reducing kettle 1) and heated to boiling.
Over a period of 32 hours, with refluxing, 600 kg of Nitro Fast Orange
GGD is run into the boiling iron-acid mixture in a very thin stream. After addition of all the Nitro Fast Orange
GGD base refluxing is continued for an additional 6 hours and the base drawn
off means of a separating device in the kettle.
Yield: 505
kg. B.P. 189℃. F.P. + 3℃.
sp.gr. 1.48.
细田豊 《理论制造染料化学》1957年。 P. 653. 5-アミノ-1,3-キシレンヘキサフルオリド. 译自PB 85172.
800 l タイル张还原釜に铁粉155 kg,水44 l,盐酸4 kgを煮沸逆流下に前记ニトロ195 kgを细流にして8-10 h かかつて装入し4 h 搅拌する。つぎに3 m3釜に洗い落して水蒸汽蒸馏すれば约1:5の割で出てくるが,初留の盐酸に完全に溶けない部分と终りのや黄味を带びてくる部分は分けて185-1900で常压蒸馏する。大部分は水と分离し,1000で亁燥し压滤過して制品とする。ファストオレンジGGDベ-ス154 kg.
张澍声 《精细化工中间体工业生产技术》1996年。 P. 138. 3,5-二(三氟甲基)苯胺。 译自BIOS 1149, 5.
在4500 L铁还原锅中加入500 kg铁屑,500 L水和20 kg乙酸,加热至沸。于32小时内600 kg硝基化合物以细流加入,加完后反应物再煮沸6小时。静置,油层取出经过压滤分离。 得505 kg 3,5-二(三氟甲基) 苯胺,沸点189℃,结晶温度3℃. 比重1.48,收率95%。
张澍声 《精细化工中间体工业生产技术》1996年。P. 156.
5-氨基-1,3-二甲苯六氟。 译自 FIAT 1313, 1, 52.
在搅拌下将44 kg水,155 kg铁粉和4 kg粗盐酸加到800 L还原锅中,加热到100℃. 在100-102℃缓缓加入195 kg硝基坚牢橙GGD(5-硝基-1,3-双三氟甲基苯),这需要8-10小时。当所有硝基坚牢橙GGD已经加入,物料用回流冷凝器再沸腾4小时,然后取样检验沉淀还原是否完全。还原完成后物料流入3000 L铁锅中。并将还原锅用热水洗涤数次。然后水蒸汽蒸馏出坚牢橙GGD色基,并很好分离。
蒸出的胺和水的比例约为1:5。 最初的一小部分并不溶解于盐酸,一般是另收的。水蒸汽蒸馏趋于末期,胺的颜色转为微黄。这一部分和最初的馏出物在常压和185-190℃进行精馏,由精馏得到的90%为GGD色基。
蒸汽蒸馏分离出的水仍带有胺的不愉快气味,这种水可用于下一批洗涤还原锅。 为了除去附着在胺上的任何痕量的水。在分离后在锅中加热至100℃,直至完全干燥。 195 kg硝基坚牢橙GGD生成154 kg坚牢橙GGD色基。
PB 70255, 7882-7903. Preparation of “Echtorange GGD base” 1938年德文生产工艺。 未抄录。(也指美国人的共享目录。)。
PB 70255
(Reel A-32) 共891页,含还原染料,色基和中间体的生产工艺。中国科学院图书馆收藏编号:MO. 3814.
PB 70422, 2072-2073. Echtorange GGD base. 德文生产工艺。未抄录。(也指美国人提供的共享资料目录。)。
PB 82232,
691. “Azoorangesalz GGD” 1946年德文生产工艺。 其中用到C.I.冰染重氮组分16. 2美元。美国人介绍如下:
From
3,5-bistrifluoromethyl aniline and 2-ethylamino-5-sulfobenzoic acid. In German.
应该是“快色素”。
国内出版物:
肖 刚 杨新玮 等编著。 《世界染料品种 – 2005年》。 全国染料工业信息中心 出版。 P.
229. C.I. 冰染重氮组分16.
基本上是原版《Colour Index》的译文,参考文献中未提及上述本人抄录的资料。(抄注: 未见引用上述历史文献!)
侯乐山 主编。 《中国精细化工产品集 – 原料及中间体 10396种》 2006年出版。
中国化工信息中心 全国精细化工原料及中间体行业协作组 出版。 P. 1129. 3,5-双三氟甲基苯胺。
产品性状: 略。 生产方法:有机化工原料中间体。
用途:该品是合成含氟除草剂和医药,染料的重要中间体。 生产厂:略。 (抄注: 未见引用上述历史文献!)
学习与思考: 抄录上述资料是否有用,请读者看吧!如果还有点用,那么我们的问题在哪里?
陈忠源 2018年7月17日 (一位历史文献义务收集,整理,抄录上网的操作工。)
文章作者:陈忠源 |