| [打印本页][打印选项] |
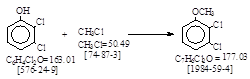
| CAS号 [1984-59-4] 生产工艺。 2,3-二氯苯甲醚 |
CAS号 [1984-59-4] 生产工艺。 2,3-二氯苯甲醚
CAS名:Benzene, 1,2-dichloro-3-methoxy- 历史参考文献:Beil. 6. E2, 178.
用途:医药中间体。LookChem网登录生产与经营单位58家。
BIOS 1153, 21a-22.(=胶卷PB 85687)。 2,3-Dichloroanisole. (Griesheim). 英国人译自德文(无资料来源)。
反应式:本人有加注。国内甲基化剂,为硫酸二甲酯,CAS号 [77-78-1]。
Summary:
2,3-Dichlorophenol
in aqueous-methanolic caustic soda aolution is reacted under pressure with
methyl chloride. After removal of the
methyl alcohol by steam distillation, the 2,3-dichloroanisole is separated as
an oil from the aqueous mixture. This is
a semi technical process not fully developed.
Process:
2,3-Dichlorophenol 500
kg. = 3.07 mol M.W. 163.
Methanol 500 kg. Caustic soda solution 400Be’ 350 kg.
Soda ash 90 kg.
Methyl chloride 250 kg.
The methanol
and caustic soda solution are charged into the autoclave, followed by the
2,3-dichlorophenol and the soda ash. The
whole is stirred cold until the phenol is converted to sodium salt as indicated
by complete solubility.
Into the cold
autoclave is now blown steadily the methyl chloride, the liquid being displaced
from the pressure cylinder in which it is contained by nitrogen. When the 250 kg. have been added the
autoclave is then heated to 110℃. a pressure of 11 atmospheres being reached. The temperature of 110℃. is maintained for 8 hours at the end of
which the pressure falls to 6 atmospheres indicating completion of the
reaction.
The contents
of the autoclave are now blown into a unit suitable for steam distillation, and
the methanol removed by blowing steam through the mixture. When all the methanol has been removed the
solution is made faintly alkaline with caustic soda and is cooled to 50℃. The
2,3-dichloroanisole is settled to the bottom of the vessel and run off to a
still.
It is
distilled under 8 mm. vacuum at 126-127℃. Yield:
479 kg. of 2,3-dichloroanisole = 2.70 mol. =88% theory. M.W. 177.
Specification: 2,3-dichloroanisole has a C.P. of 31.6℃.
张澍声 《精细化工中间体工业生产技术》 1996年。P. 62.
2,3-二氯苯基醚。 译自BIOS 1153, 21a.
2,3-二氯苯酚在甲醇 – NaOH水溶液中与氯甲烷在加压下反应。水蒸汽蒸馏除去甲醇后,2,3-二氯苯基醚以油状物从水溶液中分出。当时德国处于中试阶段,未充分开发。
500 kg甲醇和350 kg 35% NaOH 溶液加到高压釜中,随后加入500 kg 2,3-二氯苯酚和90 kg Na2CO3. 整个搅拌冷却直至2,3-二氯苯酚转变为钠盐从而完全溶解。向冷高压釜中稳定地压入氯甲烷,氯甲烷液体是装在压力钢瓶内包含氮气中。加入250 kg氯甲烷后,高压釜加热至110℃, 压力达到11巴。在110℃保温8小时,反应末期压力降至6巴,说明反应已完成。
高压釜内容物压出后进行水蒸汽蒸馏,通入直接蒸汽吹出甲醇。当所有甲醇除去后,用NaOH使溶液变为明显碱性,冷却到50℃。 2,3-二氯苯基醚沉降在容器底部,从容器中放出。在8 mmHg和126-127℃蒸馏,得到479 kg 2,3-二氯苯基醚,为理论量的88%。2,3-二氯苯基醚凝固点31.6℃。
PB 785. 2,3-Dichloroanisole. 1941年德文生产工艺。共3页。 未抄录。
侯乐山 主编。 《中国精细化工产品集 – 原料及中间体10396种》 2006年。 P. 410. 2,3-二氯苯基醚。 (无资料来源)。
生产方法:由2,3-二氯苯酚经甲基化而得。
将二氯苯酚与30% 氢氧化钠溶液一起加到反应釜中,搅拌使其溶解,在65-75℃滴加硫酸二甲酯与氢氧化钠溶液,在87℃反应1.5 h,冷却到50℃,加水后再冷却到10℃。过滤,滤饼加入到乙醚中,用无水硫酸钠干燥,过滤,滤液回收乙醚即得本品。
广告:
由沈阳院何岩彬 主编的《染料品种大全》已正式出版。 1500元。
陈忠源 2018年8月16日。
文章作者:陈忠源 |