| [打印本页][打印选项] |
| CAS号 [119-93-7] 生产工艺。 3,3’-二甲基联苯胺 |
CAS号 [119-93-7] 生产工艺。 3,3’-二甲基联苯胺
CAS名:[1,1’-Biphenyl]-4,4’-diamine, 3,3’-dimethyl- 历史参考文献:Beil. 13, 255; E1, 79; E3, 484; E4, 419。
用途:酸性红114。冰染偶合组分5, 40。冰染重氮组分113。直接黄2, 48。直接橙6, 7, 10, 13, 30, 31。直接红2, 15, 21, 22, 34。
直接红39, 56, 67, 68。 直接紫21, 28, 39。 直接蓝3, 14, 21, 25, 26, 27, 31, 39, 53, 60, 63,
231, 295。 直接绿20, 85。
直接棕52, 147, 222。 直接黑24, 30, 154。 溶剂黄107。 颜料黄77, 107。 颜料橙15, 47。
LookChem网登录生产与经营单位79家。 反应类别:还原,转位。
BIOS 986, 384-386.(=胶卷PB 77764)。 Tolidine Base
distilled. (I.G. Griesheim). 英国人译自德文,无资料来源。
反应式:本人有加注,德文原件未抄录。
125 kg. alcohol 95% , 50 kg. solvent naphtha, 300 kg. zinc dust are
heated to 35-40℃. and well agitated. 5 kg. caustic soda 50% and 80 kg.
o-nitrotoluene are then added and the mixture raised to the boil 80-82℃. Reduction is started and then
maintained by the alternate additions of caustic liquor 50% and o-nitrotoluene
to give a total addition of 20 kg. caustic soda 50% and 170 kg. o-nitrotoluene. When the reaction dies down after all the
caustic has been added and before completion of reduction to the azo stage
alcohol is distilled off and replaced with 300 kg. solvent naphtha, thus
raising the boiling point.
The resulting azo toluene is reduced to hydrazo toluene at 95℃. by the addition of 150 kg. naphtha, 70 kg. zinc, 15 l. water. After completion of reduction to hydrazo the
latter is taken as completely as possible into solution at 95℃. by the addition of 300 kg. solvent naphtha. The zinc oxide is fixed as the heavy coarse
hydroxid by the addition of 100 l. water and the batch allowed to settle. The naphtha solution is then run off through
the coarse zinc oxide via the bottom run-off and zinc oxide filter-screen to a
receiver. The zinc oxide is further
extracted at 90℃. with 300 kg. naphtha, the naphtha solution
being run-off and combined with the strong solution.
A further quantity of 300 kg. naphtha is then added for a second
extraction at 90℃. of the zinc oxide and the weak naphtha
solution run-off and used for the next batch.
The combined strong naphtha solution of hydrazotoluene are then pumped
to the conversion vat containing 100 l. water and 30 kg. sodium bisulphite 40%
liquor at 45℃. To
effect conversion. 970 kg. 22.5%
sulphuric acid is then run into the conversion vessel at 45℃. After addition of the acid
800-900 l. water is added and the batch agitated for 2 hr. at 55-60℃. it is then slowly heated to 75℃. A concentrated sodium carbonate solution is
then added with the temperature rising from 75℃. to 90℃. till the mixture is just alkaline to
litmus. After agitation the batch is
allowed to settle and the aqueous sodium sulphate layer drawn off at 90℃. from the naphtha-tolidine layer.
The naphtha layer is then washed with 600 l. water and finally allowed
to cool slowly with agitation. The
precipitated toluidine base is filtered off on the vacuum filters and washed
first with cold solvent naphtha and then with water. The filter cake is then dug off the filters
and dehydrated in the prestill at 160-170℃. The dehydrated molten tolidine is sucked over
to the final still and distilled over as rapidly as possible at a pressure of
15 mm. Hg abs. The distilled base is
blown to the cooling tray, solidified and cooled in a 4 in. layer to 30℃. approx. and finally broken out and ground.
Actual materials consumptions/tone of tolidine base distilled M. W.
212.
o-nitrotoluene 1.55 t. yield = 83% theory。 Zinc
dust 2.15 t. (Process de,ands 2.3
tones)。 Naphtha 0.28 t。 Alcohol
95% 0.78 t. recovery 0.68 t。 60%
sulphuric acid as SO3 0.7 t.
(Process demand 0.56 t/t)。 Sodium carbonate 1.0 t.
(Process demands 0.74 t/t for neutralization of sulphuric acid 0.56
t/t)。 Caustic soda 0.1 t。
Sodium bisulphite 40% 0.05 t。
Hydrochloric acid 20% 0.4 t. (For
neutralization of alkali in zinc oxide prior to incineration)。
Analytical data. 分析数据:Tolidine base
C.P. 127.5℃.
Plant: 设备:略。 Annual Production: 年产量:1937年
132,000 kg.
细田豊 《理论制造染料化学》1957年。 P. 507-508. I.G. Griesheim法。 O-トリジン. 译自PB 77764.
アゾ还原。エタノ- ル125 kg,ソルベンントナフタ50 kg,亚铅末300 kgを35-400で搅拌しながらNaOH 50% 5 kg,o-ニトロトルエン80 kgを加え沸点80-820に保ちながらNaOH 50% 20 kgとo-ニトロトルエン170 kgを少量ずつ交互加え,アゾ还原终る前にアルコ- ルを蒸馏してその代りにソルベントナフタ300 kgを加える。
ヒドラゾ还原。 アゾ还原が终つたらナフタ150 kg,Zn 70 kg,水15 l を追加し950でヒドラゾトルエンに还原する。
抽 出。ナフタ300 kgを加え950でヒドラゾを全部溶解し,水100 l を加えて静止し酸化亚铅を凝集せしめ,ナフタを底からスクリ- ンを通して拨き取り,ナフタ300 kgを用いて酸化亚铅から900で抽出し,さらにナフタ300 kgをもつて抽出しこれは次回の抽出に用いる。
转 化。 浓厚なヒドラゾトルエンのナフタ溶液は转化槽で水100 l ,NaHSO3
40% 液30 kgを450で加え,硫酸22.5% 970 kgを注加,水800-900 l を加え55-600で2 h,しだいに750に上げ,Na2CO3 浓溶液を加えてアルカリ性として搅拌後,静止して下层のNa2SO4 液を拨き,水600 l で洗い,ナフタ部は搅拌しながや冷し,トリジンベ- スの结晶を滤過,ナフタで洗いつぎに水で洗い,160-1700で脱水の後15 mmで蒸馏し,4“ 层に固ませ碎いて粉碎する。收率83%。
又是多余的说明:
1945年以后,美国人和英国人分别出版,德国生产工艺的英文译文(以美元和£标价),1957年,日本人细田豊以《理论制造染料化学》出版从美国人和英国人的英文译文出版日文。1992年《科技资料图纸征订目录》,是上海市科明设计研究所 编印。(一)国外新产品,新技术。 1. 日本染料中间体制造法及工艺规范 – 175元。实际是 – 细田豊资料的摘录本。1996年,《染料工业》编辑部出版:张澍声 编译《精细化工中间体工业生产技术》一书,主要是美国人和英国人英文译文的中文版(258元)。2014年,出版《颜料和染料生产技术》一书,实际上是美国人和英国人英文译文的中文译文(见书评 – 为什么?因为读者没有这类图书)。
2016年开始,我免费提供历史资料(当然是抄录文),有读者说《考古》,我想,至少你可以了解一些产品的历史。
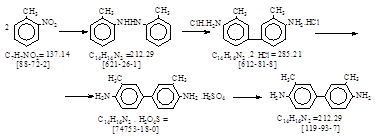
张澍声 《精细化工中间体及产品生产工艺》 2006年。 P. 456. 3,3‘-二甲基联苯胺。 译自BIOS 986, II, 94.
125 kg 95% 乙醇,50 kg 溶剂石脑油和300 kg锌粉加热到35-40℃,并很好搅拌,然后加入5 kg 50% 氢氧化钠和80 kg 邻硝基甲苯。混合物加热到沸腾温度80-82℃,开始还原。交替加入50% 氢氧化钠溶液和邻硝基甲苯,总计加入20 kg 50% 氢氧化钠溶液和170 kg邻硝基甲苯。在所有氢氧化钠溶液加完并且还原到偶氮阶段完成之前,反应速度减慢,这时蒸馏出乙醇,并用300 kg 石脑油代替,这样来提高沸点温度。
在95℃加入150 kg石脑油,70 kg 锌粉和15 L水,将生成的偶氮甲苯还原为氢化偶氮甲苯。还原完成后,加入300 kg石脑油使氢化偶氮甲苯在95℃尽可能溶解,加入100 L水使氧化锌以粗粒子氢氧化锌固定下来,反应物静置。石脑油溶液流经粗粒子氧化锌,由底部流出到接受器中。氧化锌再在90℃用300 kg石脑油萃取,洗涤后的石脑油流出与前面的浓溶液合并。再在90℃用300 kg石脑油第二次萃取,该石脑油流出后用于下批加料。合并的氢化偶氮甲苯浓溶液用泵送往转位槽,槽内含有100 L水和30 kg 40% 亚硫酸氢钠溶液,温度45℃。为了进行重排,向转位槽中加入970 kg 22.5% 硫酸(45℃)。加完硫酸后,再加入800-900 L水,反应物在55-60℃搅拌2小时,然后缓缓加热到75℃,加入浓的碳酸钠溶液,直至混合物对石蕊试纸刚好为碱性,同时温度由75℃上升至90℃。搅拌后静置,在90℃从石脑油 – 联甲苯胺层抽吸出硫酸钠水溶液层,石脑油层用600 L水洗涤,在搅拌下缓缓冷却,在抽滤机上真空过滤出沉淀的联甲苯胺,先用冷石脑油溶剂洗涤,然后水洗。滤饼从抽滤器中挖出,在160-170℃预蒸馏脱水,脱水后的熔融联甲苯胺抽吸到真空蒸馏釜中,在15 mmHg尽可能快地蒸馏出来。蒸出的联甲苯胺放入冷却盘中,固化并冷却到30℃,厚度为10 cm,粉碎并研磨。
每生产1 t 联甲苯胺消耗:邻硝基甲苯 1550
kg; 锌粉 2150
kg; 石脑油 280
kg; 乙醇(95%) 780
kg;
硫酸(60%,以SO3计) 700
kg; 碳酸钠 1000
kg; 氢氧化钠 100
kg; 亚硫酸氢钠溶液(40%) 50kg.
联甲苯胺 (3,3‘-二甲基联苯胺) 熔点 127.5℃。
BIOS 1153, 264-266.(=胶卷PB 85687)。 o-Tolidine. (Leverkusen).
英国人译自德文,无资料来源。
Process: 生产工艺:1. Reduction: 还原:
A 4000 l. well-dried reduction vessel is charged with 690 kg. of
o-nitrotoluene and 2 l. 50% caustic liquor, and heated to 800. At 80-900, 700 kg. of zinc dust (90%, theory
=658.5 at 100%) are added during 8 hours in 10 kg. portions; simultaneously
45.1 of 50% caustic liquor are run in.
After 2 hours’ further stirring the mass id diluted by the addition of
800 l. of water, the first 200 l. of which must be added during 2 hours.
Test: 测试:for o-nitrotoluene. The
azo-compound is further reduced to hydrazo by adding at 85-900 during 4-5
hours, a further 230-240 kg. of zinc (10 kg. at a time). Simultaneously with
the addition of 600 l. of water. It is
stirred with further additions of zinc if necessary until reduction to hydrazo
is complete.
Test: 测试:A sample stirred with benzene must not impart a yellow colour to the
solvent.
2. Zinc removal: 除锌:The batch is diluted to 4000 l., with water,
cooled to 20-300, and blown to the dezincing vat containing 2000 l. of water or
wash liquor from a previous batch. 30%
hydrochloric or sulphuric acid (3000 l.) is run in carefully at 15-250 so that
a brown colour is given to Congo Red paper 20 minutes after the last
addition. The hydrazo-compound is
filtered and washed until the wash liquor gives no precipitate of zinc hydroxide
on addition of sodium acetate.
3. Conversion: 转位:The hydrazo=compound from two reduction vessels is charged at 5-100
during 4-5 hours into the conversion vat containing 3000 l. of water and
1200-1300 l. of 190Be’ hydrochloric acid (= 10% hydrochloric acid). Stirring is continued until conversion is
complete.
Test: 测试:A sample is filtered, the residue stirred with benzene and the
separated benzene layer shaken with conc. H2SO4. No needles of tolidine sulphate should be
formed. 500 kg. of rock salt are added;
a sample after filtration should give no further precipitation on addition od
sodium sulphate. The batch is filtered.
4. Crystallisation: 结晶:The crude product is slurried with water and boiled. The solution (vol. 10000 l.) must be nearly neutral
(just weakly acid to Congo), otherwise any remaining zinc is not
precipitated. 1-2% of sodium sulphate is
added to remove lead, 15 kg. of terrana added, and the batch allowed to settle. The clear solution at 750 is blown to a
tiled, rubber-lined vat, and 500-800 kg. of rock salt added. After cooling to 200 and testing for complete
precipitation it is filtered and washed twice with conc. Brine.
The remaining solution in the clarification vat is left behind for a
total of four batches and is then diluted with water, boiled and blown via a
press into the vessel used for dilution of new batch.
The residue remaining is blown to drain if it contains less then the
equivalent of 6 kg. of NaNO2. Yield: 85%
theory.
抄注:Leverkusen工厂生产工艺,未见有人翻译。
FIAT 1313, I, 257.(=胶卷PB 85172)。 o-Tolidine. (I.G. Leverkusen). 美国人译自德文,无资料来源。
Charge the 4000 l. reducer with 690 kg o-nitrotoluene and 2 l. sodium
hydroxide, 50%. Heat to 80℃. and add during 8 hours at 80-90℃. in 10 k.
portions 700 kg zinc dust, 90%. Simultaneously
run in at a steady rate 45 l. sodium hydroxide, 50%. Stir 1.5 hours after all is charged. There should be no odor of nitrotoluene. Add, slowly 800 l. water, the first 1/4 takes
2 hours. Now add in 4-5 hours at 85-90℃. 240 kg zinc dust, 90%, and
simultaneously 600 l. water. Stir until
the reduction is finished, no azo compound extractable with benzene. Dilute to 4000 l. and suck to the dezincing
vat containing 2000 l. water, or mother liquor from preceding charge. Add about 3000 l. sulphuric acid, 30%, at
15-25℃. to weak Congo acidity that remains for 20
minutes. Filter and wash free of zinc
suulphate, no precipitate with sodium acetate solution. Two charges of this hydrazo paste are charged
with cooling at 5-10℃. during 4-5 hours into 3000 l. water and
1250 l. hydrochloric acid, 190Be’. Stir
at 5-10℃. until the rearrangement id complete. Add 500 kg salt. Filter but do not wash the paste. The purification is similar to Dianisidine
except that it is done at 75℃.
The yield is 85% of theory. 抄注:Leverkusen生产工艺,未见有人翻译。
PB 25624, 989-991. o-Tolidine 德文生产工艺原件,未抄录。
PB 74239. Reports on the
productions of various organic chemicals. 1932-1943年。 共661页。国内有收藏。
PB 74239, 127-129. o-Tolidine. 1940年德文生产工艺,未抄录。
国内研究动态:
朱国诚 (南通农药厂研究所)。 联甲苯胺的生产和应用。 [J] 染料工业,1985, 4, 10-16.
联甲苯胺的合成:1) 锌粉法。 2) 铁粉法及硅铁粉法。 3)其它还有路线。 联甲苯胺的应用。 本人不在抄录。
参考文献:共20篇。未见引用到上述本人抄录的文献。
陆跃进 单绍军 宋东明 王华崧 (大连理工大学国际重点实验室)。
催化加氢制备3,3’-二甲基联苯胺。 [J] 染料工业,2002, 4, 30-31. 实验部分:
在200 ml 高压釜中加入13.7 g 邻硝基甲苯,40 ml 乙醇,40% NaOH 20 g,Pd/C 催化剂0.2 g,助催化剂0.2 g,乳化剂0.2 g,封釜后用氢气置换三次,充压到0.6 MPa,分别在30℃和90℃下分段反应。在此条件下反应7.5小时。反应结束后,冷却,释压,开釜,将产物倒入一烧杯中,用少量乙醇洗涤两次,然后进行抽滤,取滤饼,加入500 ml烧瓶中,加入45 ml水,搅拌,在30℃下滴加浓盐酸30 ml,反应45分钟后,升温至90℃反应1小时后,趁热过滤,取滤液,在滤液中加入30% 氢氧化钠至pH值为9-10,过滤取滤饼,在红外灯下干燥,得产品8.4 g.
结果与讨论:略。 结论:收率>75%,纯度>97%。
参考文献:3篇。 未见引用上述文献。
国内出版物:
侯乐山 主编 《中国精细化工产品集 – 原料及中间体10396种》。 P. 378. 3,3’-二甲基联苯胺。
中国化工信息中心 全国精细化工原料及中间体行业协作组 出版 《版权所有 未经允许 不得翻印》。
生产方法:用锌粉(或硅铁粉)在氢氧化钠介质中将邻硝基甲苯还原为2,2’-二甲基对称二苯肼,然后在22.5%的硫酸及焦亚硫酸钠(或盐酸)存在下,重排生成3,3’-二甲基联苯胺硫酸盐(或盐酸盐),再加30% 液碱,脱酸而得成品。
抄注:无资料来源。
国内染料专业出版物;
何岩彬 主编 《染料品种大全》。 沈阳出版社 出版。 2018年。 P. 1993. 3,3’-二甲基联苯胺
上面抄录文可供读者参考。
【留得青山在不愁没柴烧】,能力有大小,免费服务,无需评述,过好每一天!
【锡山区 揽才放大招】- 今日无锡晚报。“人才”不是一天,二天可能形成的。是否可以多提一下基础教学(就是我们的大学)。如果我们的某所大学 – 是世界第一!到时,有一天,我们的大学,你美国人要来留学,还需我们同意!本老人是也有思想的动物!不是高材生,是普通人才,又如何!当然要奋斗!
不当之处,请大家批评!
陈忠源 2019年6月11日星期二。
文章作者:陈忠源 |