| [打印本页][打印选项] |
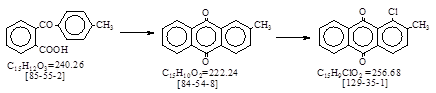
| CAS号 [129-35-1] 生产工艺。 1-氯-2-甲基蒽醌 |
CAS号 [129-35-1] 生产工艺。 1-氯-2-甲基蒽醌
CAS名:9,10-Anthracenedione, 1-chloro-2-methyl- 历史参考文献:Beil. 7. E3, 4105.
用途:还原橙9。还原红5。颜料橙40, 51。颜料红216。 LookChem网登录生产与经营单位13家。反应类别:氯化。
BIOS 987, 60.(=胶卷PB 75860)。 1-Chloro-2-methylanthraquinone. 英国人译自德文,无资料来源。
反应式:本人有加注,德文原件未抄录。
400 kg. of 2-methylanthraquinone is dissolved in 4000 kg. of 5% oleum
and 1 kg. of iodine added. The vessel is
now closed and the contents cooled to 5℃. 151 kg. chlorine are passed into the vessel
over a period of 10 hours, the chlorine cylinder being finally heated with
water to drive in the last traces.
During this time the temperature must not rise above 8℃. The agitation is continued
for a further 6 to 8 hours to enable all the chlorine to be absorbed. The mixture is now transferred into warm
water, filtered and well washed.
The products are formed during the chlorination, the
1-chloro-2-methylanthraquinone and 2-methyl-3-chloroanthraquinone. These must be separated. The mixture is stirred with 1000 litres
water, 1780 kg. sodium sulphite
(50%), 44 kg. copper sulphate, 90 kg. sodium carbonate. The mixture is heated for 15 hours at 120℃. in a closed vessel (pressure 1 to 1.5 atmospheres), cooled down,
filtered, and well washed and dried.
Yield = 275 kg. of 1-chloro-2-methylanthraquinone = 59% of
theory. M. P. 144-150℃.
The chlorine content is 12.8 to 13.5%.
A good product must be nearer to 13.5% chlorine, because the theoretical
is 13.8%.
抄注:本品主要是用于CAS号 [81-26-5] 的生产。未上网,但是1-氨基-2-甲基蒽醌法已在2016年5月18日上网。
FIAT 1313, II, 41-43.(=胶卷PB 85172)。 1-Chloro-2-methylanthraquinone. 美国人译自德文,无资料来源。
This material is manufactured at Ludwigshafen from PTB (T-Säure), this
being ring closed in sulfuric acid to 2-methlanthraquinone, which is then
chlorinated, without isolation, chiefly to 1-chloro-2-methylanthraquinone. The crude product after isolation is given a
preliminary purification with alkaline sulfite, which removes only part of
polychloro compounds present.
A brief description follows:
The ring closure of PTB is carried out in a 3.2 cubic meter steel
jacketed agitated pressure kettle and to this are charged 1437 kg. of sulfuric
acid (96%) and 1063 kg. oleum (24%) to form 2500 kg. of sulfuric acid
(100%). This acid mixture is heated to
50℃. and to it are added 500 kg. of PTB (T-Säure)
in portions. This mixture is agitated
for 2-3 hours to dissolve the PTB and then held 2 hours longer at 50℃. To this is then added 350 kg.
chloro sulfonic qcid and the temperature raised to 110-115℃. and held at that point for 1 hour.
A test at this point should indicate that ring closure is complete. The charge is then cooled to 20-25℃. and blown over to a steel kettle of similar size, equipped for
chlorination under pressure. At this
point are added 875 kg. of oleum (24% and 1875 grams of iodine. The charge is then cooled to 5-8℃, the kettle closed and 147-149 kg. chlorine is forced in under pressure
so that the internal pressure in the kettle reaches 1-2 atmospheres, the temperature
not being allowed to exceed 8℃.
When all the chlorine has been forced in, the charge is allowed to
agitate under pressure for 6-8 hours longer below 8℃. by which time all the chlorine should have been absorbed. A sample is then withdrawn from the kettle,
which should now be under no chlorine pressure and this sample should shows no
free chlorine present when tested with Indigo sulfonate solution. An isolated sample of the crude at this point
should show an organic chlorine analysis of 13.3 to 14.0%.
Into a 22 cubic meter acid proof brick lined dilution tank should then
be charged 20 cubic meters of warm water 35℃. and the
contents of the finished chlorination blown over into it. The precipitated slurry is then filtered off
into a large wooden filter press and washed neutral with hot water.
The press cakes from two batches,, manufactured as described above,
are then charged into a 22 cubic meter tank.
This tank is a pressure vessel having an alkali proof brick lining,
which contains 12 cubic meters of water and when the cakes are well mixed, to
the slurry are added 1780 kg. of sodium sulfite (50%), 890 kg. of sodium
sulfite (100%), 44 kg. copper sulfate crystals and 90 kg. sodium carbonate and
the slurry heated to 90℃. after which the kettle is closed and heated
to 120℃. and this temperature held for 15 hours, the
pressure reaching 1.2-1.5 atmospheres.
At this point the pressure is released and the vessel filled with cold
water and the material filtered off in a large cast iron filter press, washed neutral
with hot water and dried in a hot air dryer.
Yield: 470 kg. (56% purity)* = 263 kg. (100% equiv.) = 60% of theory
methylanthraquinone** = 49% of theory from PTB.
F. P. 144-150℃.; chlorine content = 12.8-13.5%.
* 1-Chloro-2-methylanthraquinone is now only manufactured for
conversion to dimethyl-dianthraquinonyl C(抄注:CAS号 [81-26-5])and hence is evaluated in the laboratory by
conversion to that product in comparison with pure
1-chloro-2-methylanthraquinone.
** Ring closure of PTB results in 89% yield of theory of
2-methylanthraquinone. (抄注:这与BIOS 987, 60中的译文有差别!)。
This reaction produces what appears to be a low yield of rather low
quality material, however, I.G. Farben, after much work, are convinced that
this material is the most economical from which to manufacture
dimethyl-dianthraquinonyl. [81-26-5].
Their reasons are as follws:
a. Based upon the 100% equivalent value of the
1-chloro-2-mthylanthraquinone present in the product, the yield of
dimethl-dianthraquinonyl is good, (over 80%).
b. The process can be carried out in simple, available equipment, the
maintenance of which is neither difficult nor expensive.
c. The nature of the impurities present is such that are readily
removed in the dimethyl-dianthraqinonyl process (Ullmann Reactin).
The impurities in the crude, before sulfite purification, are given as
2-chloro-3-methylanthraquione – 15% approx.; polychloro methylanthraquinone –
8% approx.; ash and other similar impurities – 10% approx.; Total – 33%.
The impurities after sulfite purification are estimated to be:
2-chloro-3-methlanthraquinone – 15% approx.; polychloro methylanthraquinone –
4% approx.; ash and other similar impurities – 10% approx.; Total – 30%.
In either case the 1,3-dichloro-2-methylanthraquinone present is not
considered as an impurity since it contributes to the final yield of
dimehyl-dianthraquiononyl obtained.
The above data sum up the reasons for continuation of the process
which has now been operation since 1935.
抄注:本人为什么这么抄录?请读者看,国内有那么多化工类出版物,至今未见到有报导或者翻译!
细田豊 《理论制造染料化学》。技報當 出版。 1957年。 P. 555.
1-Chloro-2-methylanthraquinone. 译自FIAT 1313.
T 酸500 kgを100% 硫酸2.5 tに500で加え2.-3 h保温して溶し,クロルスルホン酸350 kgを加えて110-1150に1 h保温して闭环し,20-250に冷し钢制の盐化机に移す。
ここで24% 发烟硫酸875 kgおよびI2 1875 gを加え,5-80に冷し密闭して盐素147-149 kgを压入する。内压1-2 气压,温度は80以下でなければならない。なお同温で6-7 h搅拌,350の水20 tに排出,滤過,湯洗する。2回分のcakeを22 m3の耐アルカリ炼瓦张加压釜の水12 tに加え,この泥状物に50% Na2SO3 1,780 kg,硫酸铜结晶44 kg, Na2CO3 90 kgを装入,900に上げ釜を闭めて120に15 h保温(1.2-1.5气压)後,压をぬき水を充して滤過,湯洗,亁燥する。 470 kg (56%) = 263 kg (100%) 收率60% / メチルアンラキノン; 49% / T 酸。
张澍声 《精细化工中间体工业生产技术》。《染料工业》编辑部 出版。非正式出版物。1996年。P. 228.
1-氯-2-甲基蒽醌。译自FIAT 1313,II, 41.
苯产品和2-甲基蒽醌在同一车间生产。对甲苯甲酰基苯甲酸在硫酸中闭环,生成2-甲基蒽醌,不经分离,直接氯化为1-氯-2-甲基蒽醌,经碱性亚硫酸钠初步精制。
在3.2 m3耐压锅中加入1437 kg 96% 硫酸和1063 kg 24% 发烟硫酸,配成2500 kg 100% 硫酸。加热到50℃,分批加入500 kg 对甲苯甲酰基苯甲酸,混合物搅拌2-3小时,使其溶解,再在50℃保持2小时。向其中加入350 kg 氯磺酸,升温至110-115℃,在此温度保持1小时,检验到表明闭环已经完成。
冷却到20-25℃,压入另一耐压氯化锅中,加入875 kg 24% 发烟硫酸和1875 g碘。物料冷却到5-8℃,密闭锅,通入147-149 kg 氯气,锅内压力达1-2巴,温度不可超过8℃。当所有氯已压入后,在8℃以下物料在压力下搅拌6-8小时,在此期间全部氯完全吸收。从锅中取样用靛蓝磺酸盐溶液检验应表明无氯存在。取样分离出粗品,分析有机氯含量应为13.3-14.0%。
将物料压入20 m3 35℃的温水中,过滤,热水洗至中性。将两锅的滤饼合并在一起,加入到22 m3 耐压槽中,槽内装有12 m3 水,很好搅拌后,再加入1780 kg 亚硫酸钠(50%),890 kg 亚硫酸钠(100%),44 kg 硫酸铜结晶和90 kg 碳酸钠。浆状物加热到90℃,闭锅,再加热到120℃,在120℃保持15小时,压力达1.2-1.5巴。然后释去压力,锅内用水充满,压滤,热水洗到中性,热空气干燥。
得到470 kg 1-氯-2-甲基蒽醌滤饼,相当于263 kg 100% 产品,以2-甲基蒽醌计,收率60%,以对甲苯苯酰基苯甲酸计,收率49%。
PB 25624, 1278-1286.
Chloromethylquinone. 2-甲基蒽醌用氯气氯化生产工艺,未抄录。
PB 25628, 3725-3727.
Chloromethyl anthraquinone from β-methylanthraquinone. 1936年7月。1美元。美国人介绍如下。
β-Methylanthraquinone is dissolved monohydrate. Chlorosulfonic acid,
oleum and some iodine are added. Then
chlorine is introduced at about 1 atmosphere gauge pressure. Dilution and neutralization follow. In German.
本人未抄录。
PB 70057, 8336-8345. Chloromethyl
anthraquinone. By Hilert. 1935年11月29日德文生产工艺,未抄录。
国内化工类产品正式出版物。
[129-35-1],本人暂未见到有翻译和报导。
张大国 编 《精细有机单元反应合成技术手册》。化学工业出版社 出版。 2014年。按分子式和中文名称,发现无报导。
【单元反应】氯化。按化合物CAS号,本人已上网如下。
[81-42-5]; [82-27-9]; [99-30-9]; [127-73-3]; [5202-86-8]; [89735-63-7]
等。
何岩彬 主编 《染料品种大全》。沈阳出版社 出版。 2018年。 P. 1998. 1-氯-2-甲基蒽醌。
陈忠源 2020年2月20日星期四。非常时期! 2020年4月1日星期三。
文章作者:陈忠源 |