| [打印本页][打印选项] |
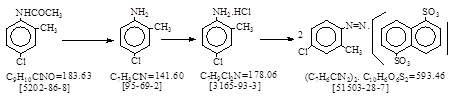
| C.I. 冰染重氮组分11(C.I. 37085)生产工艺。 CAS号 [95-69-2] |
C.I. 冰染重氮组分11(C.I. 37085)生产工艺。 CAS号 [95-69-2]
CAS名:Benzenamine, 4-chloro-2-methyl-
历史参考文献:Beil. 12, 835.
发明者:Wagner 1921年。 生产工艺参考文献:BIOS 986, 119-126.; FIAT 764 – Echtrot TR
Base; Echtrotsalz KB.
用途:冰染染料及染料中间体等。 LookChem网登录生产与经营单位83家。 反应类别:乙酰基水解。
BIOS 986, 119-126.(=胶卷PB 77764)。 5-Chloro-o-toluidine Free base and Hydrochloride salt. (Hoechst). 英国人译自德文。
反应式:本人有加注,德文原件未抄录。[5202-86-8] 已在2018年8月23日上网。
续已上网 [5202-86-8]。
The contents of“Stripper”are blown to the hydrolysis vessel by means
of a blow pipe reaching to the bottom of the latter. The hydrolysis vessel has been previously
charged with 426 kg. 32% caustic soda solution to neutralize the acidity still
present in the stripped material. The
chlorobenzol present in the alkaline mixture is driven off by distillation with
live steam, the chlorobenzene-water vapour mixture being condensed and
separated in the normal manner and the chlorobenzene pumped back to the main
storage tank via a calcium chloride drying tower. The chlorobenzene distillation takes about 10
hours. The residue in the hydrolysis
vessel, i. e. the acetyl derivative, is added. 1100 kg. 33% caustic soda
solution for hydrolysis. All joints of
the vessel are sealed and the contents heated by means of live steam (15 ats.)
to 175℃. for 8 hours to effect hydrolysis. The vessel contents are then discharged under
their own pressure to the separator (No. 7) containing 2500 litres of water
from the chlorobenzol steam distillation.
After standing for 8 hours the aqueous upper later, sodium acetate
solution, is run to drain via an interceptor and the lower layer of mixed bases
(5 and 3-chloro-o-toluidine) to the crude bases receiver. 2 chlorination batches are collected in a
receiver and blown to the Frederking stills fro pre-distillation under
vacuum. The purpose of this distillation
is to remove the non-volatile tar stc. from the crude oil before it proceeds to
the fractionating still, where removal would be more hazardous.
Aqueous distillate is collected in receiver (10) and blown back to
separator (7). The crude mixed bases
distilled over are collected in the crude bases storage tank (11). The residue in the Frederking stills is mixed
in the stills with Kieselguhr, stirred well and cooled and then removed by
shovel.
The crude oil charged under vacuum to the fractionating still (12) in
operations of 10 M3 and distilled, with the addition of 25 kg. of sodium
acetate for three distillations. The
residues after fractionation are removed occasionally by blowing to a remote
sump.
o-Toluidine distills over first, followed by chloro-toluene,
3-chloro-o-toluidine, 5-chloro-o-toluidine and di-chloro-o-toluidines.
In a typical distillation the fractions are approximately:- 1. o-toluidine - 600 kg.
Reflux ratio 10 to 1。 2.
Intermediate product - 100 kg.
Reflux ratio 12 to 1。
3. 3-chloro-o-toluidine - 700 / 800 kg. Reflux ratio
10 to 1。 4. Intermediate product -300 / 400 kg. Reflux ratio 12 to 1。
5. 5-chloro-o-toluidine -5,500 kg.
Reflux ratio 2 to 1。
6. Intermediate product - 400 kg.
Reflux ratio 5 to 1。
The various intermediate fractions are of course re-distilled in the
normal manner and the final yield per chlorination batch is:-
642 kg.
5-chloro-o-toluidine.; 86
kg. 3-chloro-o-toluidine.; 52 kg.
di-chlroo-toluidine.; 66 kg. o-toluidine.
Analytical data: 反应中各类化合物的分析数据:
1. Acet-o-toluidine: C .Pt. 108 to 109℃. Acetic acid – 0.2%; Water –
0.2%; o-toluidine – 0.2%.。
2. 5-chloro-o-tolidine: C. Pt. 26
to 27℃.
Boiling point – 240℃. at 760 mm.; Nitrite value – 100%.。
4. o-toluidine: C. Pt. – 24℃.; Specific gravity – 1,004 at 15℃.; Boiling point – 200℃. at 760 mm.。
5. 2,5-di-chloro-o-toluidine:
C. Pt. 52℃.; Boiling point – 260℃. at 760 mm.。
Stage 2. Preparation of
hydrochloride Salt: 5-氯-2-氨基甲苯盐酸盐:
3400 kg. Methylene chloride or chloroform is charged to the salt
formation vessel and 800 kg. 5-chloro-o-toluidine base added. Hydrochloric acid gas is then introduced
above the surface of the solution, cooling and stirring during the
process. After the completion of the
salt formation the thick slurry is discharged to the dryer and the solvent
distilled off via 2 lead coolers to the storage vessel (2) for solvent. Finally the dryer is put under vacuum to
remove the last traces of solvent. 30
kg. NaHSO4 is added to bind any dissociated base and the mass cooled with
continued stirring. The dryer is emptied
by agitator via the side hole which is covered with flange cover during
drying. The product is discharged to
drums.
Completion of salt formation is tested for by adding concentrated
hydrochloric acid to the filtrate from a sample withdrawn from the vessel when
there should be no desposition of the hydrochloride. The product contains 97 to 98* hydrochloride
salt. The 5-chloro-o-toluidine used
should have a C. Pt. of not less than 26.5 to 27℃.
Yield: 1,00 kg. of salt per
batch, equivalent to 784 kg. 5-chloro-o-toluidine 100% M. W. 141.5 = 98.2%
theory. based on 5-chloro-o-toluidine (M. W. 141.5) charged.
Summarized Yields:
5-chloro-o-toluidine hydrochloride 100% as base M. W. 141.5 = 66.3%
theory.; 5-chloro-o-toluidine free base
100% M. W. 141.5 = 67.5% theory.;
3-chloro-o-toluidine free base 100% M. W. 141.5 = 9% theory. The above yields, based on acet-o-toluidine,
do not allow for o-toluidine recovered fron fractionation and re-cycled. The strong hydrochloride discharged from the
dryer was diluted with sodium chloride to 91% hydrochloride in the standard
manner.
Miscellaneous Notes: 以下含设备能力,操作周期,历年产量和收率,公用工程单耗,设备明细表等:略!
细田豊 《理论制造染料化学》。技報當 出版。 1957年。P. 509-510. 5-クロル-2-トルイジン(ファストレッド TR ベ-ス).摘译文。
(3)加水分解:15 m3铁釜のNaOH 33% 426 kgに排出し,クロルベンゼンを水蒸汽蒸馏した後NaOH 33% 1.1 tを加え15气压の生蒸汽を通じて1750に8 h保温し,静置して上层の酢酸ソ-ダ液を分ける。
(4)蒸馏:まず蒸馏しないタ-ル质等を除くために真空蒸馏した後分馏してつぎの馏份とする。o-トルイジン 66 kg,混合ジクロルトルイジン52 kg。 5-クロル-2-トルイジン 642 kg收率67.5% 对アセトトルイジン。 3-クロル-2-トルイジン 86 kg, 收率9.0%
(5)铅ホモゲン釜でメチレンクロリドまたはクロロホルム3.4 tに5-クロル-o-トルイジン800 kgを加え,搅拌しながらHClガスを表面に通じ盐酸盐の泥状物となし,蒸馏器で溶媒を蒸馏しなお减压で蒸馏する。解离したベ-スを结合するためにNaHSO4 30 kgを加え横口から取出す。纯度97 – 98%,收率98.2%,市贩品はNaClで91% 浓度にする。
张澍声《精细化工中间体及产品生产工艺》。沈阳院 出版。2006年。P. 493-494.
5-氯-2-氨基甲苯基盐酸盐。译自BIOS 986, 119.
续已上网 [5202-86-8]。吸收器中物料压送至水解槽中,其中预先加有426 kg 32% 氢氧化钠溶液,以中和仍然存在的酸度,碱性混合物中的氯苯用直接蒸汽水蒸汽蒸馏出来,将氯苯 – 水混合物用通常方法分离,氯苯经氯化钙干燥塔回到主贮槽中,氯苯蒸馏约需10小时。水解槽中留下的是乙酰衍生物,加入1100 kg 33% 氢氧化钠溶液进行水解,所有连接点密封,用15 kg压力的直接蒸汽加热。在175℃加热8小时使水解完成,利用其自身压力压送到分离器中,分离器中预先放有2500 L由氯苯水蒸汽蒸馏分离的水。放置8小时后,上层的醋酸钠水溶液流入中间收集器中。下层的混合胺(5- 和3-氯邻甲苯胺)流到粗胺接受器中,接受两锅的氯化物后,压送至Frederking 蒸馏塔,在真空下进行预蒸馏。该蒸馏的目的是从粗胺中除去不挥发的焦油,如果在精馏塔中除去会更危险。
蒸馏出的水经接受器送往分离器,蒸馏出的粗混合胺送入贮槽。Frederking 蒸馏的残留物在釜中与硅藻土混合,很好搅拌并冷却,用铲除去。粗胺在真空下加到精馏釜中,加入25 kg 醋酸钠。首先蒸出邻甲苯胺,随后是氯代甲苯,3-氯-2-氨基甲苯,5-氯-2-氨基甲苯和二氯-2-氨基甲苯。
典型的精馏馏份大体是:(1)邻甲苯胺600 kg,回流比10 :1;(2)直接产物100 kg,回流比12:1;(3)3-氯-2-氨基甲苯700 – 800 kg,回流比10:1;(4)中间产物300 – 400 kg,回流比12:1;(5)5-氯-2-氨基甲苯5500 kg,回流比2:1;(6)直接产物400 kg,回流比5:1。
在正常情况下各中间馏份当然再精馏。每锅氯化物的最终收率是:642 kg 5-氯-2-氨基甲苯,86 kg 3-氯-2-氨基甲苯,52 kg 二氯-2-氨基甲苯混合物,66 kg 邻甲苯胺。
各项产品和原料规格:乙酰基邻甲苯胺:结晶温度108 – 109℃,醋酸0.02%,水0.2%,邻甲苯胺0.2%。 5-氯-2-氨基甲苯:结晶温度26 – 27℃,沸点240℃(760 mmHg),亚硝值100%。 3-氯-2-氨基甲苯:结晶温度9 – 9.4℃,沸点220℃(760 mmHg),亚硝值100%。 邻甲苯胺:结晶温度 – 24℃,比重1.004(15℃)沸点200℃(760 mmHg)。 2,5-二氯邻及苯胺:结晶温度52℃,沸点260℃(760 mmHg)。
(二)5-氯-2-氨基甲苯盐酸盐的制备:
在成盐槽中加入3400 kg 二氯甲烷或氯仿,再加入800 kg 5-氯-2-氨基甲苯。将氯化氢气体加到溶液的表面上,在加入时冷却并搅拌。在完成成盐后,稠厚的浆状物排放到干燥器中,蒸馏出溶剂,然后抽真空,将最后的微量溶剂除去。加入30 kg 硫酸氢钠,以结合所有离解的胺,在继续搅拌下将物料冷却,将产品经过侧孔排放装桶。产品含有97 – 98% 盐酸盐。所用5-氯-2-氨基甲苯的熔点不低于26.5 – 27℃。每784 kg 100% 5-氯-2-氨基甲苯生成1000 kg 盐酸盐,以5-氯-2-氨基甲苯计,收率98.2%。
以乙酰邻甲苯胺计,不考虑邻甲苯胺的回收和循环,各项产品的总收率是:5-氯-2-氨基甲苯盐酸盐66.3%,5-氯-2-氨基甲苯67.5%,3-氯-2-氨基甲苯 9%。
PB 25629, 286-289.
4-Chloro-o-toluidine. 产品德文分析方法,未抄录。
PB 25625, 385-393. “Echtrotsalz TR”1933年稳定重氮盐德文生产工艺。= [51503-28-7] 生产工艺。美国人介绍如下。1美元。
5-chloro-2-toluidine hydrochloride is diazotized. The product is coupled with
naphthalene-1,5-disulfonic acid. A
complete description of the entire process is given. An alternative method starts with the
diazotization of 5-chloro-2-toluidine which is also described. In German.
【抄注】这一资料可能国内读者没有见过。当然,在今天来说,已是过时了!
PB 70150, 539-555.
5-Chloro-2-toluidine. 德文生产工艺,未抄录。
PB 70361, 6880-6882. Echtrot
TR-Reinbase (4-Chloro-o-toluidine = Fast Red TR pure Base).
美国人介绍是:1940年2月6日德文生产工艺。1.5美元。本人未抄录。
PB 73377, 2478-2484.
5-Chloro-2-toluidine. 这是美国人的调査报告,未抄录。
PB 74025, 1011-1014. Echtrot TR
Base. 本人未抄录。
章思规 主编《精细有机化学品技术手册》。科学出版社 出版。1991年。 # 10030. 5-氯-2-氨基甲苯。 本人未收藏。
侯乐山 主编《中国精细化工产品集 – 原料及中间体10396种》。2006年。P. 890. 4-氯邻甲苯胺。摘录。
中国化工信息中心 全国精细化工原料及中间体行业协作组 出版 《版权所有 未经允许 不得翻印》。
【生产方法】主要是 [5202-86-8] 本人已上网。
【用途】钠盐,医药和染料的重要中间体。 【生产厂】3家。
何岩彬 主编 《染料品种大全》。沈阳出版社 出版。 2018年。P. 393-394.
C.I. 冰染重氮组分11。
陈忠源 2020年5月10日星期日。
文章作者:陈忠源 |