| [打印本页][打印选项] |
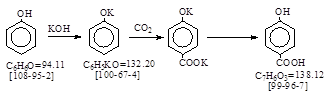
| CAS号 [99-96-7] 生产工艺。 对羟基苯甲酸 |
CAS号 [99-96-7] 生产工艺。 对羟基苯甲酸
CAS名:Benzoic acid, 4-hydroxy- 历史参考文献:Beil. 10, 149; E1, 68; E2, 38; E3, 277; E4,
340.
用途:食品添加剂,化妆品,医药和农药等。 LookChem网登录生产与经营单位203家。反应类别:羧化。
BIOS 986, 219-227.
4-Hydroxybenzoic acid (Technical). (I.G. Offenbach). 英国人译自德文,无资料来源。
张澍声《精细化工中间体工业生产技术》。《染料工业》编辑部 出版。1996年。 4-羟基苯甲酸。译自BIOS 986, 219.
反应式:本人有加注,德文原件未抄录。(本人同时抄录中文译文,供读者参考!)
Use: The purified product is used as a food preservative.
Preparaed by the action of carbon dioxide on dry potassium
phenolate. 其反应方程式不再重复抄录。
Phenol Balance: 苯酚的物料平衡:of 3000 kg. charge of phenol = 1644 kg is
converted to 4-hydroxxybenzoic acid + 1065 kg. is recovered + 291 kg is lost. =
总量3000 kg.
Material consumptions: 投料量:(抄录不再分项)。
3000 kg. Phenol。 1814 kg. Potasch 100% as 50% liquor (i. e. 1.5% excess)。 1440
kg. Carbon dioxide。 3640 kg. Hydrochloric acid
22.5%。 10 kg.。
7 kg. T.O. carbon。 0.2 kg. Hydrosulphite。 15 kg. Bisulphite liquor 40%。
Yield 2412 kg. 4-hydroxybenzoic acid 100% M. W. 138. Yield = 85% theory or phenol consumed (3000
loss 1065 recd. = 1935 kg.).
A. Potassium Phenolate. 苯酚钾盐的制备。
1. Formation。制备。
1814 kg. KOH 100% as 50% liquor is run into the carbonation kettle and
3000 kg. phenol is then added. After
addition of the phenol the vessel is closed and the batch heated to 120 – 130℃. internal pressure 1.2 – 1.5 ats: heating steam 20 ats.
【苯酚钾的制备】将1814 kg 100% 氢氧化钾制成50% 溶液,加入待羧化锅中,然后加入3000 kg 苯酚,关闭羧化锅,加热到120 – 130℃,压力1.2 – 1.5巴。
2. Drying. 干燥。
This stage proceeds in a similar manner to the drying of sodium β-naphtholate
(BON acid) using pressure release,“initial drying”, and“final drying”
systema. At 130℃. the pressure release cock is opened slowly so that the pressure
falls to zero in 2.5 hr. With continued
heating the internal temperature rises to 183℃. in 10 hr. With heating still
on the carbonation vessel is now connected to the“initial drying”receiver and
vacuum system and in the course of the application of vacuum the temperature
falls to 145 – 150℃. The
batch is heated to an internal temperature of 165℃. When it is connected to
the“final drying”system at pressure 15 mm. Hg. abs. The contents are finally dry at 195℃ drying time 24 hr.
【干燥】苯酚钾的干燥与2-萘酚钠的干燥采用相似的方法,分为压力释放,最初干燥和最后干燥阶段。在130℃缓缓打开压力释放阀,于2.5小时内压力降至零。继续加热,于10小时内内温上升到183℃,于继续加热羧化的同时,将羧化锅与最初干燥接受器及真空系统连接,在抽真空过程中温度降至145 – 150℃。当其与最终干燥系统连接,压力为15 mmHg时,反应物加热到内温165℃。反应物在195℃最后干燥24小时。
B. Precarbonation. 预羧化。
With full heating steam on the carbonation vessel carbon dioxide is
admitted for about 20 min. to the closed vessel to combine with the small
amount of excess potash. Water formed in
this reaction is distilled off by the heat of reaction and jacket steam in
about 1 – 1.5 hr. a little phenol also distils over. Final temperature 205℃. It is essential that in no
case should CO2 addition start below 180℃. below this
temperature salicylic acid is formed.
【预羧化】在充分加热下向羧化锅内通二氧化碳20分钟,与少量过量的氢氧化钾反应。反应生成的水于1 – 1.5小时内由反应热蒸馏出来,少量苯酚也蒸馏出来,最终温度205℃。必须注意,不可在180℃以下通入二氧化碳,低于此温度会生成水杨酸。
C. Carbonation. 羧化。
1st carbonation. The vacuum cock is shut and carbon dioxide is
admitted via a 2.5 mm. crifice. In order
to keep the temperature below 220℃. the steam
is shut off after 1.5 hour. The full
pressure of 4 – 5 ats. is reached after 7 hours from start of CO2 flow and
maintained for a further 2 – 3 hours.
The internal emperature falls somewhat to 215℃. in the last hour. At
temperatures 200 – 220℃. some decomposition of 4-hydroxybenzoic acid
occurs, to phenol and carbon dioxide, hence the prescribed maximum temperature
of 220℃.
【羧化】1. 第一次羧化:关闭真空,通过2.5 mm板孔流进二氧化碳。为了保持温度在220℃以下,1.5小时后停止加热。通二氧化碳7小时后达到4 – 5巴的满压,再继续通2 – 3小时,在最后1小时内温略降至215℃,4-羟基苯甲酸发生一些分解,生成苯酚和二氧化碳,因此限制最高温度为220℃。
1st phenol distillation. After
completion of the 1st carbonation the pressure is let off and distillation of
phenol started via the“initial drying”vacuum receiver. When distillation slows down the batch is
connected to the main (higher vacuum) naphthol distillation pump. Temperature at end of distillation 190℃. pressure 15 mm Hg. abs. total
time 6 hours.
【第一次苯酚蒸馏】完成第一次羧化后,释放压力,并经过最初干燥真空接受器开始蒸馏苯酚。当蒸馏驱缓时,反应物与较高真空度的泵连接,蒸馏最终温度190℃,压力15 mmHg,总时间为6小时。
2nd Carbonation. Carbon dioxide is admitted via the 2.5 mm. crifice
plate, full pressure is maintained for 2 hr.
Higest temp. 220℃.
【第二次羧化】经过2.5 mm孔板通入二氧化碳,在满压保持2小时,最高温度220℃。
2nd Phenol distillation. As foe 1st distillation, final temperature
180℃. Time 7 hours.
【第2次苯酚蒸馏】与第一次苯酚蒸馏相同,最终温度180℃,时间7小时。
3rd Carbonation. Carbon dioxide
is introduced via the 2.5 mm. crifice and absorption is often very energetic
initially, particularly if the 3nd carbonation was sluggish. However, by hand manipulation of the controls
a pressure of 4.5 ats, can be schieved with a temperature not exceeding 220℃.
【第三次羧化】通过2.5 mm孔板加入二氧化碳,吸收在开始是非常费力的,特别是第二次羧酸盐为粘滞的。但是用手调节控制器,在不超过220℃的温度下,压力可以达到4.5巴。
3rd distillation. In the course
of the distillation the temperature falls to approx. 165℃. time 5 hours. Total occupation
of carbonation vessel 85 hours.
To end of drying operation 24 hours.
To end of precarbonation 27 hours.
To end of 1st carbonation 37 hours.
To end of 1st distillation 43
hours.
To end of 2nd carbonation 53 hours.
To end of 2nd distillation 60 hours.
To end of 3rd carbonation 81 hours.
To end of 3rd distillation 87 hours.
Should one of the carbonations be very sluggish a 4th carbonation and
distillation can be’ carried out but in general this is not necessary.
The recovered phenol is melted from the receiver and measured and
should be approximately 35% of the charge and is recycled.
【第三次蒸馏】在蒸馏过程中,温度降至约165℃,时间5小时。总的羧化工艺时间85小时。达到干燥结束为24小时。达到预羧化结束为27小时。达到第一次蒸馏结束43小时。 达到第二次羧化结束为53小时。达到第二次蒸馏结束60小时。达到第三次羧化结束81小时。达到第三次蒸馏结束87小时。第4次羧化应当是非常粘稠的,但一般说来不需要进行第4次羧化和蒸馏。回收的苯酚接近加料量的35%。
D. Lixiviation. 浸滤。Lixiviation of the carbonation mass is
carried out below 100℃. as alkaline solutions of 4-hydroxybenzoic
acid decompose on boiling. Water, or
mother liquor from the purification stage recrystallisation is run into the
evacuated carbonation vessel up to the level of the top blade of the agitator;
temperature falls to about 80 – 90℃. After stirring some time the crude solution
is discharged from the carbonation vessel via a blow-leg. The vessel is then half-filled with water or
mother liquor from the crystallization, the contents agitated and blown
out. The vessel is then clean and ready
for the next batch.
【浸滤】羧化物的浸滤是在100℃以下进行,因为4-羟基苯甲酸的碱溶液在沸腾时分解。将水或精制阶段重结晶的母液加到排放的羧化锅中,加到搅拌器最高桨叶的位置,温度降至约80 – 90℃。搅拌一段时间后,粗溶液从羧化锅中压出。然后锅内充满一半的水或结晶母液,搅拌并吸出锅内物,清洗羧化锅。
E. Isolation of crude
4-hydroxybenzoic acid. 粗品4-羟基苯甲酸的分离。
The work-up operations are undertaken in metal-free plant. The crude lixiviation solution is diluted
with 2000 l. water to 210Be’. And the main part of the alkali neutrallised with
hydrochloric acid. To avoid decomposition
the above neutralization should be carried out as soon as possible after
lixiviation. 8 m3 of the weakly alkaline
solution is blown to the neutralization tank, dilute with water to 140Be’, and
hydrochloric acid added to end point of faint red on litmus.
7 kg. T. O. charcoal, 15 kg.
bisulphite solution 40% are then added to the faintly acid solution. 10 kg. zinc dust is now sprinkled in and the
batch is held at 60℃. for 1 hour.
The batch is now filtered at 60℃. through
press (5) to the precipitation tank. If
necessary the filtrate is further brightened with an extra 200 gm.
hydrosulphite. The 4-hydroxybenzoic acid
is now precipitated at 60℃. with hydrochloric acid, and the batch
cooled to 15℃.
After cooling to 15℃. the batch is blown to the vacuum filter or
centrifuged and either“Paddled”or“whizzed”as dry as possible.
Vacuum filtered product : 65% solids.
Centrifuged product : 83% solids.
Note : Removal of iron can also be carried out with Na2S although loss
effectively than with zinc dust. If
sodium sulphite is used the neutralized solution is treated at 60℃. with 40 kg. Na2S, 50 kg.
Bisulphite liquor 40%, 20 kg. T. O.
carbon (from the recrystallisation) and filtered at 60℃.
Treatment with zinc dust gives a superior product and sulphide
treatment has been abandoned. The moist
product is dried on aluminium or Haveg trays in 3 air circulation ovens at 100℃. in 30 hours. The dry product
should be as bright as possible.
Purity 99.5 – 99.8%. Salicylic acid nil.
M. Pt. 216 – 217℃.
【粗品4-羟基苯甲酸的分离】在不含金属的设备中进行。粗浸滤溶液用2000 L水稀释至比重1.17,碱的主要部分用盐酸中和,为了防止分解,在浸滤后上述中和应尽可能立即进行。8000 L弱碱性溶液压入中和槽中,用水稀释至比重1.107,加盐酸至石蕊试纸为微红色即为终点。
然后将7 kg活性炭,15 kg 40% 他硫酸氢钠溶液加到此弱酸性溶液中,喷洒10 kg 锌粉,反应物在60℃保持1小时,并在60℃压滤到沉淀槽中,必要时再用200 g 保险粉增白。4-羟基苯甲酸在60℃用盐酸沉淀,并冷却到15℃。抽滤或离心过滤,尽可能抽干,抽滤产物含固量65%,离心产物含固量83%。
【注】铁的除去也可用硫化钠进行,但是效果比锌粉差。如果使用硫化钠,则中和后的溶液在60℃用40 kg 硫化钠,50 kg 40% 亚硫酸氢钠溶液和20 kg 活性炭处理,并在60℃过滤。用锌粉抽滤得到优质产品,因而硫化钠法已被放弃。
湿产品在铝盘中于100℃干燥30小时,在空气循环箱中进行。干产品尽可能白亮,不含水杨酸,纯度99.5 – 99.8%,熔点216 – 217℃。
Plant : 设备:(抄录不再分项,无译文)。
1. Carbonation vessels: as for
2,3-hydroxynaphthoic acid。 2. Neutralisation tank : 15 m3,
rubber-lined M. S. with“Quirl”agitator。
3. Press- rubber covered wooden
plates and frames。 4. Precipitation vessel : 15 m3
rubber-lined M. S. hallow shaft 3. Agitator (batch blown through shaft) lead
cooling coil with connections for water and brine。
5. Centrifuge or vacuum filter,
rubber-lined and covered。 6. 3 air circulation ovens, aluminium or Haveg
trays。
Items 2,3,4,5 are in a separate building (Fast Salts Plant). Item 6 is in the main drying shed T. 4.
Note : this product appears to have been taken over from Leverkusen
during the war – approx. 1942.
Control Tests:
1. Before lixiviation a sample
is taken from the carbonation vessel.
100 gm. is dissolved in 400 cc. water at room temperature and neutralized
with 16% hydrochloric acid to weak red on litmus. 1 gm. zinc dust, 1 gm. T. O. carbon are now added and the
solution filtered. 5 cc. Bisulphite 40%
liquor is now added and the solution brightened with a little hydrosulphite and
the 4-hydroxybenzoic acid precipitated with hydrochloric acid at ordinary
temperature. The acid is filtered at 10℃. washed and dried. The dry
product should have purity 99.8% and yield should be 60 – 64 gm. Anything less than 60 gm. is regarded as
poor. This is not a routline test.
2. A complete analysis of the
crude carbonation mass is made at intervals and a good batch should be ;-
Dipotassium salt 52.5% (= 33.8% free acid, i. e. 48% Kolbe
reaction). Monopotassium salt 44.5% (=
34.9% free acid, i.e. 52% Schmitt reaction).
【抄注】Kolbe reaction (Kolbe electrolysis). The synthesis of organic compounds by
oxidative electrolysis and decarboxylation of organic salt.
Schmitt synthesis. See
Kolbe-Schmitt carbonation. The preparation
of aromatic hydroxyl acids by carbonation of phenols by CO2 in the presence of
strong bases.
Phenol 0.8%. K2CO3
0.8%. 4-Hydroxybenzoic acid by
precipitation 64%.
The higher the carbonation temperature the more unfavourable becomes
the ratio between the di- and mono-potassium salts, i.e. the proportion of
dipotassium salt increases and that of monopotassium salt falls. The most favourable conditions for the Schmitt
reaction appear to be a temperature of about 200℃.
3. The distillate from the
phenolate drying is measured and examined.
The“final drying”in general yields no condensed distillate. During the“initial drying”about 60 litres of
condensate are obtained, containg 3.8 kg. phenol, from a charge of 9000 kg.
phenol. The distillate in the pressure
release phase amounts to 2600 l. containing 16.1 kg. phenol, for 9000 kg.
phenol charge. Total distillate
collected = 2660 l. (19.9 kg. phenol) from a charge of 9000 kg. phenol.
4. Mother liquor from vacuum
filter in filtration of precipitated acid contains 0.15% - 0.2% phenol.
5. Determination of presence of
salicylic acid in technical quality 4-hydroxybenzoic acid : 1 gm. of dry acid
is dissolved in 1000 cc. hoy water, 100 cc. of the solution is diluted with 100
cc. water and 15 cc. FeCl3 solution (0.4%) added with dilution, water to 250
cc.
A violet colour shows the presence of salicylic acid.
4-hydroxybenzoic acid (purified). 4-羟基苯甲酸的精制。
The technical product is recrystallised from water in metal-free
plant.
Materials : 800 kg. 4-hydroxybenzoic
acid (technical) 100% in the form of vacuum filter or centrifuge paste. 1 kg. zinc dust, 10 kg. T. O. carbon, 3.2 kg.
bisulphite 100% as 40% solution, hydrosulphite (small amount).
Either mother liquor from a previous recrystallisation or well water
(filtered) is used for solution purposes : the mother liquor can be reused in
not more than 3 subsequent batches and is then used for lixiviation of the
crude carbonation mass.
6000 l. water or mother liquor is charged to the solution vessel
followed by 800 kg. 4-hydroxybenzoic acid 100%.
The mixture is agitated and 1 kg. zinc dust, 10 kg. carbon T. O. are
then added. The temperature is raised to
95℃. with live steam and the batch diluted to
8000 l. with water. It is then filtered,
by CO2 pressure, through a heated press backed up by a ceramic guard candle
type filter : the filtrate runs to the crystallisation tank. 3.2 kg. bisulphite
100% as 40 liquor is now added and the batch cooled to 10℃. using brine if necessary, in summer.
If necessary a little hydrosulphite is added to brighten the
crystalline material before blowing to the filter. The product is filtered on a vacuum filter,
washed and“paddled”and sucked as dry as possible. Dry on aluminium trays in two hot water
heated vacuum stoves at 90 – 95℃. 24 hours.
The product can also be dried on Haveg trays in air circulation ovens at
90 – 95℃. 30 hours.
Mill in an“Express”mill.
Yield 752 kg. 4-hydroxybenzoic acid pure = 94% theory.
【4-羟基苯甲酸的精制】精制是在无金属设备中从水中重结晶。
800 kg 100% 工业4-羟基苯甲酸(以真空滤饼或离心滤饼状态)溶解于6000 L水(可以用上次重结晶的母液,也可以用新水,母液重复使用只能用3次,3次以后用与粗羧化物的浸滤)中,混合物进行搅拌。然后加入1 kg锌粉,10 kg 活性炭,将混合物用水稀释到8000 L,在涂有防护蜡的压滤机中过滤,滤液流入重结晶槽中加入3.2 kg 100% 亚硫酸氢钠制成的40% 溶液,冷却到10℃。必要时在过滤前加入少量保险粉以增白物质。抽滤,洗涤,尽可能抽干,在铝盘中于90 – 95℃干燥24小时。
得752 kg纯的4-羟基苯甲酸,收率94%。
Plant: 设备:(抄录不再分项,无译文)。
1. Solution vessel. 1 X 15 M3
rubber-lined M. S. pressure vessel.
Rubber covered agitator and steam post : all fittings rubber-lined. Pipes to press – aluminium。
2. Press. Solid rubber or rubber
covered wood. (some deterioration occurs with solid rubber)。 2 A.
Guard press. Multi-candle type
filter. During the war great difficulty
was experienced in obtaining reliable filter elements and the candle-type
filter had been abandoned in favour of a small completely rubber-covered M. S.
vac. filter approx. 2 ft. 6 in. X 3 ft. square.
It was gathered that operation of the multi-candle type filter had never
been very satisfactory due to frequent breakages of individual candles and the
plant mamager was in favour of a design incorpotating individual candles fed
from a header so that any particular broken element could be isolated。
3. Crystallisation vessels. 2 X 4 M3 enamel C. I. vessel: rubber-covered
stirrer with hollow shaft; rubber-lined pipes to nutsch. Jacket for heating and cooling。
4. Nutsch. Rubber-lined M. S. Jute and cotton cloth. 2 X 15 M3 rubber-lined vacuum receiver。
5. 1 X 15 M3 rubber-lined M. S.
receiver for filtrate from press 2。
6. Vacuum oven or air oven。
7. Express mill。
Production of 4-hydroxybenzoic acid pure: 精制4-羟基苯甲酸历年产量:
1942年:205,000 kg. 1943年:232,000 kg.
1944年:154,000 kg.
Special test for 4-hydroxybenzoic acid pure:
The product must be colourless after suspension in boiling water and
soluble to extent of 10% in water (with the molar equivalent of NaOH) 10 gm. is
boiled with 60 cc. water for odour test.
40 cc. of the correct strength caustic liquor is then added for the
caustic solubility test. It shall also
be soluble without residue or colour in alcohol – 10 gm. product to 25 cc.
alcohol.
【抄录说明】英国人有耐心把德文翻译成英文,我应该如实抄录英文译文供读者参考!
PB 25623, 256. 4-Hydroxybenzoic
acid. 本人未抄录。
PB 74051. 4-hydroxybenzoic
acid. 本人未抄录。
细田豊《理论制造染料化学》。技報當 出版。 1957年。 P. 477. P-Oxybenzoic acid. 译自PB 74051.
フェノ-ル1800 kgとKOH 50% 2177 kgを1800 まで蒸发後20 mmで200 - 2100 まで亁燥する。1850 に冷しCO2 を压入し4.5气压,1900 で3 h反应後,压を拨きフェノ-ルを真空蒸馏する。つぎに220 - 2300 で再びCO2 を压入し15 – 16 h後15 气压の蒸汽加热を30 气压にかえて热すること4 hで再びフェノ-ルを蒸馏し,さらにCO2 を2 hで压入,3 h搅拌後またフェノ-ルを蒸馏する。装入したフェノ-ルの35 – 36%を回收する。
徐克勋 主编《有机化工原料及中间体便览》。辽宁省石油化工技术情报总站 出版。 1989年。P. 491-2.
对羟基苯甲酸。
【制法】酚钾羧化法。
【成盐】将40% 左右的氢氧化钾溶液加入反应罐中,再加入苯酚,于100℃搅拌反应半小时,至酚钾液的游离碱在0.3 – 1.2%。即可升温进行常压脱水,至内温为140℃时改为减压脱水,直至内温170℃/93325帕以上,加入溶剂酚共沸脱水,得含水酚,干燥终点为内温达180 – 200℃/93325帕以上,即得酚钾与酚的复合盐。
【羧化】将上述复合盐继续加热至220 – 230℃通入净化无水二氧化碳,维持压力在0.49兆帕,反应2.5小时,温至200℃补加酚,保温搅拌30分钟,然后抽真空回收酚。再经二次羧化后,冷却至180℃,加水溶解,即得羧化液。
【酸析】将硫酸逐渐加入羧化液中,于60 – 80℃中和至pH = 6 – 7为止,冷却过滤,除去硫酸钾,即得成品。
章思规 主编《精细有机化学品技术手册》。科学出版社 出版。 1992年。 # 21720. 对羟基苯甲酸。本书本人未收藏。
侯乐山 主编《中国精细化工产品集 – 原料及中间体10396种》。2006年。P. 275. 对羟基苯甲酸。
中国化工信息中心 全国精细化工原料及中间体行业协作组 出版。《版权所有 未经允许 不得翻印》。
【生产方法】苯酚钾羧化法较适于工业化生产。
将40% 左右的氢氧化钾溶液与苯酚加入反应锅中混合,于100℃搅拌0.5 h,至酚钾的游离碱为0.3 – 1.2%。加热,进行常压脱水,至内温为140℃时改为减压脱水,在10.6 kPa压力下蒸水约0.5 – 1 h,直至内温达170℃以上。加入溶剂苯酚共沸脱水,至200℃(2.67 kPa)结束脱水,得酚钾与酚的复合盐。
将制备的上述复合盐继续加热至220 – 230℃,通入净化无水的二氧化碳,压力维持在0.5 MPa,反应2.5 h,降温至200℃补加苯酚,保温搅拌30 min,然后减压回收苯酚至尽。再通入二氧化碳继续第二次羧化,约需2 h。羧化结束后,回收苯酚,冷却至180℃乙酰,加水溶解,即得羧化液(对羟基苯甲酸二钾盐)。
将硫酸逐渐加入羧化液中,于70℃以下中和至pH为6.7,冷却过滤除去硫酸钾,所得粗品滤饼用水重结晶,活性炭脱色,即得含量99% 以上的对羟基苯甲酸。
【生产厂】11家。
张大国 编著《精细有机单元反应合成技术手册》。化学工业出版社 出版。2014年。P. 135.
# 022187. 对羟基苯甲酸。
【制法】A法。配料比:苯酚 :氢氧化钾(40%):溶剂苯酚 :二氧化碳 :补加苯酚 : 二氧化碳 : 水 = 1 : 0.61 : 0.5 : 1.09 : 0.5 : 0.47 : 2。
将40% 氢氧化钾溶液与熔融的苯酚混合,控制内温不超过100℃,搅拌30 min。取样化验游离碱为0.1 – 1.2%(否则应补料,直至合格),得苯酚钾溶液。将苯酚钾溶液投入反应罐,先常压蒸水,待内温升至140℃时减压蒸水,压力10.3 kPa,约1 h,大部分水蒸出后,再继续减压至170℃(2.5 kPa)时,水基本蒸尽,加热至190℃,加入溶剂苯酚共沸蒸馏带水,至200℃(2.58 kPa)停止带水,加热至200 – 230℃通入净化的二氧化碳,羧化2.5 h,至不吸收为止。降温至190℃,补加苯酚,保持温度180 – 190℃,搅拌30 min。在220℃(2.58 kPa)减压回收苯酚至尽,停止回收。于210 – 240℃下搅拌通入净化的二氧化碳,二次羧化约2 h。放掉二氧化碳,减压回收苯酚,待内温达230℃(2.58 kPa)时,停止回收,降温加水溶解,得对羟基苯甲酸二钾。搅拌,控制内温不超过70℃,逐渐加入60% 硫酸溶液至pH = 6.7,冷至50℃,过滤,水洗,合并滤液,洗液,逐渐加60% 硫酸至pH = 2,冷至10℃,过滤,水洗,甩干,得粗品。用5倍蒸馏水重结晶,加活性炭脱色,得对羟基苯甲酸。收率约65%。
B法。水杨酸转位法,不再抄录。(抄注:两种方法,均无资料来源!)。
【抄注】我为什么要化时间这么抄录英文,日文和国内的出版物?而且已不是第一次,唯一希望是:读者理解本人的用意,如有不当之处,也请读者谅解!我不仅仅是“老有所乐”,而是希望“老有所为”能做点力所能及的小工作!
陈忠源 2020年8月19日星期三。
文章作者:陈忠源 |