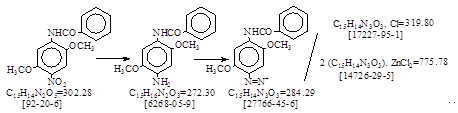
C.I. 冰染重氮组分24(C.I. 37155)生产工艺。 CAS号 [6268-05-9]
CAS名:Benzenamine, 4-(benzoylamino)-2,5-dimethoxy)- 历史参考文献:Beil. 13. E3, 2170.
发明者:Glietenberg, Neelmeier, Rimele 1928年。
Colour Index介绍生产工艺:BIOS 1149, 54-60; FIAT 764 – Echtblau RR
Base; Echtblausalz RR.
用途:染色,颜料紫25。LookChem网登录生产与经营单位:色基15家;重氮体4家;重氮体氯化物2家;稳定重氮盐26家。
BIOS 1149, 57-60.(=胶卷PB 80376)。4-Benzoylamino-2,5-dimethoxyanilide. (Leverkusen). 英国人译自德文,无资料来源。
反应式:本人有加注,德文原件未抄录。
Process: 操作步骤(粗品生产):
The vessel is charged with 8000 l. of pyridine water containing 2900
kg. of pyridine, and 1500 kg. of 4’-nitro-2’,5’-dimethoxybenzanilide equivalent
to 200 kg. NaNO2 is then added. The
mixture is heated to 950 and held 2 hours.
1600 l. of 40% bisulphite solution is added and the temperature
maintained at 950 for another 6 hours.
During 4 hours, 80 kg. of zinc dust are added and the batch is then held
at 950 for 4 hours to complete reduction.
440 l. of 30% caustic liquor are run in (weakly acid to litmus) and
the pyridine is distilled off during 12 hours, about 8000 l. being collected
(charge for next batch).
The pyridine-free charge is diluted with 10000 l. hot water, cooled to
600 and blown to the nutsche. The
nutsche cake is washed sulphate-free and pilled as dry as possible. Yield = 1000 – 1200 kg. act. wt. ≈175 kg.
NaNO2 =87.5% theory.
4-Benzoylamino-2,5-dimethoxyaniline = Fast Blue 2R Base.
Outline: 粗品精制概要:
Crude 4-benzoylamino-2,5-dimethoxyaniline is dissolved up as the
hydrochloride, screened from slime and the product reprecipitated with soda.
Materials: 投料量:
Crude 4-benzoylamino-2,5-dimethoxyaniline ≈88 kg. NaNO2 (=0.5 of a
crude batch).; Hydrochloric acid 19.50Be’. 500 l.;
Soda ash (as conc. Solution) 200 kg.;
Esbit-Entfärbungs kohle (carbon). 20 kg. (抄注:这是脱色用活性炭); Sawdust.
20 kg.
Plant: 设备:(抄录不再分项)。
1 Vat, 13000 l. capacity, iron, rubber and acid-resisting tile-lined. Propeller agitator of wood。 1 Vat, 13000 l. capacity, rubber-lined and
tiled with Asplit. Propeller agitator of
wood。 1 Centrifugal pump, rubber-lined, bronze
vanes。 1 Storage vessel, rubber-lined, 500 l. (for
HCl)。 1 Pressure vessel of iron, 10000 l. (for soda
solution)。 1 Iron press, 90 X 65 cm.
rubber-lined, wooden frames, double covered with jute。 1
Nutsche, 5 cb. m. Iron, rubber-lined with wooden base covered with asphalt. Jute
cloth。 1 Nutsche 5 cb. m. Iron, rubber-lined. Tiled
bottom with Asplit cement。 1Vacuum receiver, 13000 l.
tiled。
1 Centrifuge, rubber-lined。 1
Drying oven。
Process: 操作步骤:
The acid-resisting vat is charged with 7500 l. of water, heated with
direct steam to 800, and 500 l. of hydrochloric acid, 19.50Be’ added. Crude 4-benzoylamino-2,5-dimethoxyaniline
(0.5 batch, 1300 kg. paste ≈ 88 kg. NaNO2) is now charged in as quickly as
possible. Care must be taken to see
that, although the reaction is always acid, at the end it must only give a
brown colour on Congo paper. As soon as
solution is effected (Sample), 20 kg. decolourising carbon and 20 kg. sawdust
are added to coagulate and blind the slime (Sample). Cold water is added to cool to 700 and the
batch pumped by means of a rubber-covered centrifugal pump through a rubber
press into a seond rubber-lined and tile (Asplit) vat, containing 2000 l. tap
water and a little soda. Simultanesusly
with this solution there is run in, with good agitation, a sodium carbonate
solution. To obtain a good sandy form of
product the reaction must show carbonate alkalinity throughout but at the end
of the addition must be acid to litmus.
Both the quality and the physical form of the pure product is dependent
on this exact procedure.
After good agitation for 10 minutes, a sample is tested in the
laboratory for (a) complete precipitation and (b) zinc content.
(a) 1 l. is filtered, the filtrate separated, and the solid then
washed with water. 0.6 l. of filtrate
(without the water wash) is boiled with diluted acid to remove bisulphite
remaining from the reduction and estimated with nitrite. It must taken only 0.1 – 0.15 g. NaNO2. If the filtrate take more nitrite, then more
soda must be added to the batch, but with great care, as an excess of soda
would precipitate zinc from the reduction and the pure base would contain much
impurity. Addition of sodium acetate
solution to the filtrate should give only a slight turbidity as the zinc remains
in solution; excess of soda on the other hand also precipitates zinc.
(b) The test for zinc content is carried out as follows: 20 g. of moist base are heated with
hydrochloric acid and a little water and diazotized as concentrated as
possible. A clear diazo solution must be
formed. If zinc is present, then the
zinc chloride double salt of the diazo-compound is precipitated. If this is the case the batch must be treated
with 10 – 20 l. hydrochloric acid to make it more acid. It must then be re-tested as described above.
When the laboratory sample is satisfactory, the batch is worked up in
the usual way. It is dropped to the
nutsche, washed with water, pulled dry, hyrdo-extracted and dried at 50 – 600.
The solution vat contains the residue of sawdust, carbon and tar. It is left for the working up of the second
half of the crude batch and if necessary for a further batch. Eventually it must be worked as follows: 5000 l. of water and some hydrochloric acid
are added, boiled with agitation for a short time, cooled by addition of water
to 700, run through the press and the product precipitated with soda as
before. The residue in the vat is boiled
up again and dropped to the nutsche under the vat for disposal.
Yield = 800 kg. pure product moist (100 = 18.5 NaNO2) ≈ 148 kg. NaNO2
= 84% theory.
Quality: Nitro-content not to exceed 0.4 – 0.6 mol. - %.
After drying and grinding it gives 600 kg. 94% product (100 ≈ 23.8
NaNO2). ≈ 143 kg NaNO2. The dried and
ground product is standardised to 90% with rock salt and sold under the name of
Fast Blue 2R Base.
FIAT 1313, I, 30.(=胶卷PB 85172)。 4’-Amino-2’,5’-dimethoxybenzanilide. (I.G. Mainkur). 美国人译自德文,无资料来源。
Reduction in 30% pyridine at start adding sodium bisulfite solution
(40%) equal to 10 times the nitrite value of the base. Finish with zinc dust 4/10 times the nitrite
value.
Neutralize to weak litmus acid with sodium hydroxide. Separate the pyridine layer, distill off
remaining pyridine from water layer.
Cool layer, distill off remaining pyridine from water layer. Cool to 60℃, filter. Yield: 88% of theory as crude base.
Recrystallize crude base from water plus hydrochloric acid to brown
test on Congo paper. Temperature 90℃. The amount of hydrochloric
acid is determined by pretest, sulfate and sulfite must be absent or they precipitate
the base. Filter at 80℃ into water and just enough soda to precipitate the base without
precipitating the zinc. Cool to 50℃, filter wash with water. Dry
at 50 – 60℃. Yield: 87% of theory.
细田豊《理论制造染料化学》。技報當 出版。1957年。P. 659. 4-ベンズアミド-2,5-ジメトキシアニリン(ファストブル-2Rベ-ス)
13 m3タイル张のピリジン2.9 tを含む水8 m3に前记ニトロペ-スト2.9 t (NaNO2 200 kg相当) を加え950で2 h保温後NaHSO3 40% 液1.6 m3を加え950で6 h保温,亚铅末80 kgを4 hで加え(リトマス弱酸性)ピリジン水约8 m3を蒸馏回收する。つぎに湯10 tを加え,600で滤過水洗する。ケ-ク1.1 t = NaNO2 175 kg相当,收率87.5%。
【精制】13 m3ゴムおよび耐酸タイル张器に冰7.5 tを入れ生蒸汽で800に热し,19.50Be’盐酸500 lを加え粗ベ-ス1.3 t (NaNO2 88 kg相当) を手速く加え最后にコンゴ-纸に褐色を呈するようにする。脱色炭20 kg,锯屑20 kgを加え,水を加えて700にしゴムプレスづ滤し,水2 m3(少量のソ-ダ)に落し同时にNa2CO3 200 kgを浓溶液として加えアルカリ性を保つが最後はリトマス酸性になるようにする。滤過水洗し50 – 600で亁燥,粉碎する。600 kg (94%),收率81%,NaCl で90% 品に标准化する。
张澍声 《精细化工中间体及产品生产工艺》。沈阳院 出版。2006年。P. 380-382.
4’-苯甲酰氨基-2’,5’-二甲氧基苯胺,译自BIOS .
在13 m3衬砖搅拌锅中加入80000 L吡啶水(含2900 kg吡啶)和1500 kg 4’-硝基-2’,5’-二甲氧基苯甲酰苯胺(相当于200 kg 亚钠量),混合物加热到95℃,在95℃保持2小时。加入1600 L 40% 亚硫酸氢钠溶液,再在95℃保持6小时。于4小时内加入80 kg 锌粉,反应物在95℃保持4小时,以完成还原反应。
流入440 L 30% 氢氧化钠溶液(对石蕊试纸为弱酸性),于12小时内蒸馏出吡啶,约收集到8000 L,用于第二批加料。
不含吡啶的物料用10 m3水稀释,冷却到60℃,送往抽滤。滤饼洗涤至不含硫酸盐,尽可能抽干。得到1000 – 1200 kg 4-苯甲酰氨基-2,5-二甲氧基苯胺,相当于175 kg 亚钠量,收率87.5%。
【精制】粗品4-苯甲酰氨基-2,5-二甲氧基苯胺以盐酸盐溶解,除去杂质过滤,滤液再用碳酸钠沉淀。精制后得到商品Fast Blue 2R Base.
在13 m3铁锅(橡胶和耐酸砖衬里,木制螺旋桨搅拌器)中,加入7500 L水,直接蒸汽加热到80℃。加入500 L 30% 盐酸,尽可能快地加入半批粗品4-苯甲酰氨基-2,5-二甲氧基苯胺(1300 kg 滤饼,相当于88 kg 亚钠量)。必须要注意观察,虽然反应总是呈酸性,但在最后仅使刚果红试纸为棕色。一旦全部溶解,加入20 kg 脱色活性炭和20 kg 锯屑,凝聚和粘合粘泥状杂质。加入冷水冷却到70℃,用衬橡胶的离心泵将反应物打到橡胶压滤机,滤液流入另一13 m3橡胶和砖衬里的锅中,锅内预先放有2000 L 水和少量碳酸钠。在滤液流入的同时,在很好搅拌下流入碳酸钠的溶液,以得到好的沙粒状产物。整个过程始终显示碳酸盐碱性,但在加料最后必须对石蕊呈酸性。纯产品的质量和物理状态取决于这一实际操作。
精制物料搅拌10分钟后,取样,于实验室分析:(a)沉淀完成状况;(b)锌含量。(抄注:译者未译!)。
锅内溶液含有锯屑,活性炭,焦油等残留物。它留待与第二个半批粗产品一起处理,必要时还可增加一批。其处理方法如下:加入5000 L水和一些盐酸短时间沸腾搅拌,加水冷却到70℃,压滤,产品如前用碳酸钠沉淀。锅内残留物再煮沸,抽滤后弃去。
得到800 kg 纯品4-苯甲酰氨基-2,5-二甲氧基苯胺滤饼,相当于148 kg 亚钠量,收率84%。
硝基物含量不超过0.4 – 0.6 mol%。经干燥和研磨后,得到600 kg 94% 产品,相当于143 kg 亚钠量。干燥和研磨后的产品加入岩盐标准化,使其含量为90%,以商品名Fast Blue 2R 销售。(抄注:这是译自BIOS 1149, 54-60)。
张澍声 《精细化工中间体工业生产技术》。《染料工业》编辑部 出版。 1996年。P. 38-39.
4’-氨基-2’,5’-二甲氧基苯甲酰苯胺。
在吡啶中还原,开始加入40% 亚硫酸氢钠溶液,等于胺的亚硝值的10倍。最后用锌粉,为亚硝值的0.4倍。用氢氧化钠中和至对石蕊为弱酸性,分开吡啶层,由水层中蒸出其余吡啶,冷却到60℃,过滤,粗胺收率88%。
加盐酸至对刚果红试纸为棕色,从水中将粗胺重结晶,温度为90℃,盐酸的数量经预先试验确定,必须没有硫酸盐和亚硫酸盐,否则它们将胺沉淀,而不沉淀锌。冷却到50℃,过滤并水洗,在50 – 60℃干燥。收率87%。(抄注:这是译自FIAT 1313, I, 30).
PB 70062, Nr. 499.
4-Benzoylamino-2,5-dimethoxyaniline. 产品分析方法,未抄录。
PB 70422, 2038-2039. Fast Blue
Salt RR. By Prosiegel. 1.5 美元。1937年10月14日重氮盐德文生产工艺,未抄录。
PB 82232, 688. “Azoblausalz RR”. 2美元。1946年重氮盐德文生产工艺,未抄录。
PB 82233,235-239. “Echtblau RR Base”. 2美元。 重氮色基德文生产工艺,未抄录。
何岩彬 主编 《染料品种大全》。沈阳出版社 出版。 2018年。P. 399. 和 p. 2002.
P. 399. C.I. 冰染重氮组分24。可增补上述内容;p. 2002. 可合成的染料:C.I. 冰染重氮组分24;C.I. 冰染重氮组分40;C.I. 颜料紫25.
陈忠源 2020年6月15日星期一。













