C.I. 碱性红1 (C.I. 45160) 生产工艺 CAS号[989-38-8]
CAS名: Xanthylium, 9-[2-(ethoxycarbonyl)phenyl]-3,6-bis(ethylamino)-2,7-dimethyl-,
chloride. 参考文献:Beil. 19. E3/4.4301.
发明者:
Bernthsen, Schmid and Rey 1892年。用途: 纺织品用,非纺织品用,含化妆品,效果颜料,激光染料等。
生产工艺文献:
BIOS 959,12, 37-41. (=胶卷 PB 63858) Rhodamine 6G Extra. 英国人译自德文,但未指明资料来源。抄录如下。
反应式: 本人有加注。它是摘译自PB 70135, 894-903 (原工艺共10页),译文不完整。请参见C.I. 碱性紫10 生产工艺。
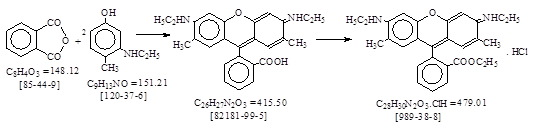
(a) Rhodamine
6G Base (=Rhodamine 2C Base) 抄注:[82181-99-5]
Materials usages per batch:
Aethylkresol rein (德文) 1,000 kg. Phthalic anhydride pure 1,300 kg. Sulphuric acid 600Be’ 1,220 kg.
Caustic soda 500Be’ @100%
550 kg.
Theoretical Yield: = 1,370 kg. Actual Yield: = 1,230 kg. Yield
% theory: = 90.
Recovery: 640 kg. phthalic anhydride dist.
Process Description:
The plant is similar to that used for Rhodamine B
Extra. 抄注:设备与碱性紫10相同,见BIOS 959,35-6.
125 kg. Monoethyl-o-amino-p-cresol is charged into the
Frederking apparatus, 50 kg. Phthalic
anhydride added and stirring commenced as soon as the charge is molten.
Make 10 additions each of 125 kg. phthalic anhydride (1,300 kg.
total) at 10-15 minute intervals whilst the temperature is rising to 1500C. At 1500C shut
off the steam, and add 750 kg.
aethylkresol during 15 min. Allow the temperature to rise to 1700C. When the first
reaction is over (1/2 hr.) add a further 125 kg.
aethylkresol. During 15 min. add 220
kg. Sulphuric acid 600Be’, and hold at 1700C for 21/2 hr. from the time
the temperature again reaches 1700C. Run the completed condensation into a mixture at 450C of 1,200 kg. caustic soda 500Be’ and
about 5,600 l. water with strong
stirring. In succeeding batches use 6,000
l. of the washings from a previous batch in place of 5,600 l. water. The caustic soda liquor suspension of the base is ground through the Konus- and Memagmuhle but unlike the B Extra Base it is simply collected in
a C. I. tank and filtered on a press. The filtered base is washed thoroughly with water and dried. 注: Aethylkresol 德文= 3-乙氨基对甲基苯酚 Konusmuhle 德文,是研磨机。
Phthalic acid recovery is carried out as the case of B Extra, but there is no
o-dichlorobenzene extraction of either the alkaline base liquors or the acid
colour liquors, these liquors being ditched.
(b) Rhodamine
6G Extra: 抄注:[989-38-8], 其未成盐体:[3373-01-1]= C28H30N2O3 = 442.55.
Materials usages per batcg: Fw. Rhodamine 2C Base 600 kg.
Ethyl chloride 200 kg. Alcohol 120 kg. Magnesium oxide 16 kg. Sodium acetate
anhydride 2 kg. Hydrochloric acid 30% 50 kg. Salt 100% 800 kg.
Process Description:
Charge 500 kg.
fresh alcohol 94% or regenerated alcohol
92%, 300 kg. Rhodamine 2C Base, and 8 kg. magnesium oxide with agitation..
Add 100 kg. ethyl chloride. The pressure is then 4 ats., and after 1/2 hr. stirring at ordinary temperature the pressure is released from 4 ats to remove air from the autoclave
and so prevent explosion. Then heat
to 100-1100C, hold for 2 hr. at that temperature, and stir without steam heating overnight. The temperature
has then fallen to 900C. Heat
to 1200C and stir for 6 hr. at that temperature. The pressure initially is 7-8 ats but falls back to 5-6
ats. Cool overnight by blowing air through
the jacket. Next morning release the pressure and draw the mixture at 65-700C into the still.
Distill off 200 l. alcohol, and then
continue the distillation running in as much water as distillate to maintain
the mass in the still mobile and stirrable. Altogether 1,050-1,100 l. is distilled off. Run the still residue into 5,000 l. water in a vat, and wash
through with a total of 500 l. water. Next day heat the contents of the vat to boiling, and add a second batch from a
still. Dilute the boiling solution with water to about 13 cu. M. and add 2 kg. sodium acetate anhydrous to remove unesterified base. 抄注:unesterified base = Rhodamine 2C Base.
Test:
Filter a sample and add a little 10%
sodium acetate solution. Filter, and
dilute with hot water. There should be
no precipitated of unesterified base.
Stand overnight, and filter first through a Spitsfilter and then through a box nutsche into a vat. The residue in the first vat is boiled
with 1,500 l. water and sucked on to the
nutsche and washed. The filtrate is transferred to the first vat for working up
with the next batch.
To the filtered colour solution at 900C add 50 kg. hydrochloric acid 200Be’ and
about 2,250 l. salt water (= about 800
kg. salt).
Note: The
salt solution usually reacts alkaline and the degree of alkalinity must be
estimated in the laboratory. Add the requisite amount of acid to correct (usually 10-20 l. hydrochloric acid 200 Be’ per 5,000 l. solution).
The colour settles out in the form of dark green
crystals. Test for complete precipitation after the addition of
salt. Cool to 700C and
Filter, the
filtrate running to waste. Leave the colour on the filter overnight and suck
off well the following day.
Dry on the Trockenpfanne
at 1200C with stirring – 6
hr. total drying time. As soon as the colour starts to become powdery add 1 kg. Phenolphthalein (Erkennungsmittel). Any
colour recovered from the vapour off-take is returned to the process at the
solution stage. Sieve and grind in a Perplex
Mill. Test for dryness by drying a sample at not more than 900C for 6 hr.: the moisture content shall not exceed 1%.
Yield: 642 kg. Rhodamine 6G Extra (Pure content is
96%). = 616 kg. pure
colour. = 107% ex. Rhodamine 2C Base. = 88.9% theory.
The solution residues
– 2,000 kg. from 20-40 batches are stirred with 5,000 l. water and
heated to 500C with
steam, and about 250 kg. caustic soda of strength 500Be’ added
until the liquor is alkaline. Heat for 2
hr. with agitation and see that the alkalinity is maintained. Filter next
day through a press and wash well with water. Dry the base in a jacketed pan and esterify in the normal manner. 90% of the colour in the residues in obtained
as good quality Rhodamine 6G Extra. 抄注:这是残液回收染料Colour.
The recovered alcohol is regenerated in the Benzol Dist. Plant. The loss of alcohol is 20 kg.
per 100 kg. Rhodamine 2C Base = 60 kg. per batch = 12% v. E. alcohol.
Solubility: Dissolve 50 kg. dry ground colour in one litre hot water with stirring and filter. Wash the
filter with 2 l. water. The residue
Should not exceed 0.5 g.
Note on the plant:
The esterification is carried out with 2 x 1,400 l.
cast steel autoclaves fitted with enamel liners. Ethylchloride is blown infrom a cylinder. Heating is by means of a
steam jacket and 5 ats steam
pressure. The gland is packed with asbestos string and graphite, the agitator
revolving at about 20 r.p.m. and
temperature measurement being with a thermometer in the centre of the agitator
shaft.
日文摘译。 细田豊 《理论制造染料化学》 1957年。 P. 789-790. ロ-タ”ミン6G エキストラ (IG)1) 1)BIOS 959. 请见原书。
PB 25626, 1402-6. Fanalrosa. Rhodamin 4GD. 1931-1934年8月生产工艺。1美元。 未抄录。
PB 25626, 1701-2. Rhodamin 6G extra. 1937年12月生产工艺。 1美元。 未抄录。
PB 70135, 894-903. Rhodamin 6G extra. 1944年11月生产工艺规程。(共10页)1.5美元。未抄录。
PB 82021, 3038-47. Rhodamin 2C Base. Rhodamin 6G extra. 生产工艺规程。(共10页)未抄录。
用途:化妆品:DFG. Kosmetische Faerbemittel / Colour for Cosmetics. 1984. C-WR Rot 15. (2pp.)
激光染料:Mitsuo Maeda. Laser Dyes.
1984. P. 86-91. # 94.
母体用途: 母体, 它的CAS号[3373-01-1]。 分子式,分子量 =
C28H30N2O3 = 442.55.
颜料:颜料红81;81:1;81:5;81:6;169. 溶剂染料:溶剂红36;79。
墨水用:Valifast
Red 1308 ; 1355; 1388 (碱性红1 + 酸性黄23)。 Valifast Red 1320 (碱性红1 + 酸性黄42) [72927-76-5]
未酯化体用途: CAS名:Xanthylium, 9-(2-carboxyphenyl)-3,6-bis(ethylamino)-2,7-dimethyl-, CAS号[82181-99-5]
染料:碱性红1:1。 颜料:颜料红81:2;81:3;81:4. 溶剂染料:溶剂红229.
加注:日本译文比我们早,我国是利用了BIOS,但二者都未利用德文资料原件,我本人看过PB报告,它比英国人的译文好多了,
有的还有错,有的是一些关键点未译,加上译者理解不同,会有差别,所以建议利用原件。以上抄录文,供参考!
陈忠源 2016年8月23日 于 无锡 明辉国际。













