C.I. 冰染重氮组分7 (C.I. 37030) 生产工艺 CAS号 [99-09-2] 橙色基R.
CAS名: Benzenamine, 3-nitro-, 参考文献: Beil. 12, 638, E1, 345, E2, 374, E3, 1541, E4, 1589.
发明者: Winther, Laska, Zitscher 1911年。
用途: 冰染染料和C.I. 分散黄5。 C.I. 酸性橙18。C.I. 酸性蓝29。C.I. 酸性棕92。C.I. 直接红169。C.I. 媒介黄1.
C.I. 媒介蓝29。 C.I. 硫化棕19。
生产工艺文献: 原版Colour
Index. FIAT 764 – Echtorange R
Base. Echtorangesalz R. 以下未本人收录的资料。
BIOS 986, 268-271. (=胶卷PB 77764) m-Nitroaniline. I.G. Offenbach. 1940年8月15日。英国人译自德文。抄录如下。
反应式: 本人有加注。译者未注明译自哪个德文原件,PB报告。
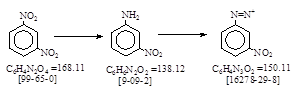
Principle: m-Dinitrobenzene is reduced with sodium
hydrogen sulphide in the presence of magnesium sulphite.
Process details:
A. Reduction:
300 kg. m-dinitrobenzene “flaked” is sucked into the
reduction vessel from a cask, together with 2876 l. water. 80 kg. magnesium
sulphite crystals are then added and the whole heated to 740C. by
means of the steam coil. 150 kg. NaHS 100% in the form of 30-40% aqueous solution and diluted to 1580 l. with water
at 200C. is prepared in a measure vessel and this 9.5% solution is
run into the reducer over a period of 20 minutes through a distributor. The
temperature rises to 940C. due to the exothermic reaction. After a
further 20 minutes agitation the reaction is stopped by the addition of 900 l.
cold water, and the whole cooled to 400C. by means of the coil.
The batch is blown to the filter, the reduction kettle
washed out with water, the washings going to the filter and the crude material
is washed on the filter as free as possible from alkali, using 3 cu. m. of
excess mother liquor from a previous recrystallisation. The filtrate and
washings from the nutsch go the drain.
B. Recrystallation:
12 cu. m. of mother liquor from a previous
recrystallisation and the same amount of water are charged to the solution
vessel (2) and the crude product from two reductions (i.e. equivalent to 600
kg. dinitrobenzene) is added. 15 kg. of Carboraffen (as material with 33% moisture)
is added, the air expelled by steam and steam then blown into the closed vessel
over the surface, for a period of 20 minutes and the batch then stirred for a
further period of 20 minutes. The pressure should be 0.4 ats., temperature 1050C.
The solution is then filtered through a Scheibler type
filter into the cooling tank and there cooled to 400C. The
recrystallised m-nitroaniline is filtered on a vacuum filter. Mother liquor
from this filtration is collected in the vacuum receiver and used for the next
recrystallisation. The excess or mother liquor arising from the condensation of
live steam during the solution process goes to a storage tank and is used for
washing the crude m-nitroaniline. Scheibler 是一种滤清过滤器。
The carbon residue from the Scheibler filter is
re-extracted with mother liquor. The crystallized m-nitroaniline is dried on
enamel or aluminium trays in avacuum oven heated with water at 950C.
the dried product being ground in a Simplex Perplex mill.
The yield from 300 kg. m-dinitrobenzene crude.(i.e.
290.1 kg. 100%) = 210 kg. 100% m-nitroaniline equivalent to 88.2% theory.
Pure m-dinitrobenzene has been reduced by the above
process and the product purified by recrystallisation and from this the average
purity of technical m-dinitrobenzene has been taken to be 96.7%. The above
yield of 88.2% theory is based on 100% m-dinitrobenzene.
Analytical data for m-nitroaniline.
1. Strength by nitrite I.G. Std. not less than 99%
2. purity is determined by coupling after
reduction
a) o-nitroaniline by reduction and
condensation with phenanthraquinone I.G.
Std. 0.5 mol%
b) p-nitroaniline
by reduction to p-phenylenediamine and determination of the latter by reduction
of siver chloride to silver. 0.5 mol%.
3. 2,4-dinitroaniline by weighing insoluble
residue from the diazotization. 4. M-nitroaniline I.G. Std. 99 mol%.
5. C.P. 111.60C. Limiting
values. 1100C. 6. Insoluble in hydrochloric acid. I.G. Std. 0.05%. Limiting values 0.2%.
Plant:
(1) Reduction vessel. M.S. vessel 6 cu.m. with
cover. Iron gate agitator, 52 r.p.m. M.S. coil for heating and cooling.
(2) Recrystallisation tank. Horizontal M.S.
cylindrical vessel, 16 cu.m. with axial paddle type agitator.
(3) Cooling tanks: (Two alternative tanks) (a)
M.S. vessel 20 cu.m. with M.S. cover and fume pipe. Cooling coil 18 sq.m. gate
type agitator or (b) M.S. pressure vessel 18 cu.m. with gate type agitator and
vacuum cooling by means of steam ejector.
(4) Filters: (a) Nutsch for crude product. (b)
Nutsch fro purified product. (c) Scheibler filter for carbon screening,
capacity 34 cu.m. with 7 filter frames.
Annual productions: 1937 115,000 kg. 1943 15,000 kg.
PB 25626, 1707-1709. Method for producing m-nitroaniline. 1938年7月生产工艺。1美元。 未抄录。
美国人介绍:This substance, also called “Echtorange R base” is produced from m-dinitrobenzene by means of sodium
hydrosulfide.
Detailed directions are given. In German. 注:未译成英文。
PB 70422, 1863-1870. 共8页。Metanitraniline (“fast orange
R base”) 1940年8月15日德文生产工艺 2美元。未抄录。
PB 70422, 2092-2093. 共2页。Fast orange salt R
(=MNA-DS) 1938年7月18日德文重氮盐生产工艺。1.5美元。未抄录。
PB 70423, 2880-2891. 共12页。Echt orange R base 1940年德文生产工艺 未抄录。
PB 74239, 118-122. m-Nitroanilin (Echtorange R base) 共5页。1940年德文生产工艺。未抄录。
中文摘译文。张澍声,《精细化工中间体工业生产技术》 1996年。P. 1. 摘译自BIOS 986,268-271. 请见原书。
译者未知有德文原件。
上海染料生产工艺汇编。《间硝基苯胺》1976年。P. 20. 抄录如下。
1. 多硫化钠制备:
将已粗碎的硫化钠290公斤加入溶解锅内,加水加热溶解,调整体积至1800升,取样按分析结果加硫黄(以100%计)137.9
公斤,在950C搅拌一小时,静置30分钟,过滤。
2. 还原;
在还原锅内加水4000升,加热至850C,搅拌下加入间二硝基苯(以100%计)600公斤和硫酸镁(以100%计)200公斤,升温至900C,保温搅拌15分钟,降温至850C,开始逐渐加入多硫化钠进行还原,加料时间3-3.5小时,加料期间,不时以醋酸铅溶液 滴于滤纸上与反应液作润圈试验,(二润圈相遇处,呈暗黑色反应表示硫化钠过剩),检验多硫化钠过剩情况,最后取样用丙酮, 液碱鉴定终点(一般呈棕色时为终点),冷却至300C,放料抽滤,滤饼用套液清洗二次,即得粗制品。
3. 精制:
将精制滤液放入精制釜内,边搅拌,边升温到800C,加粗制间硝基苯胺,加完后用盐酸调整pH值至6.5-7,密闭精制釜升温
至1020C,搅拌20分钟使溶解,静置45分钟,上层澄清液放入冷却桶,同时立即加入重亚硫酸钠,察看pH值。用碳酸钠或盐酸
调整pH值至6.5-7,在搅拌下冷却至350C,抽滤,其滤饼即为间硝基苯胺及制品。 得量约409公斤。
国内研究动态:
李春梅 陈亚庆 刘彭兰。硝基苯胺衍生物的气相色谱分析。 [J] 染料工业,1997, 2, 31-34. 请看原期刊。
刘硝智 等和 张 欣。硒催化还原间二硝基苯制备间硝基苯胺的研究。[J] 染料与染色,2014, 4, 43-45. 摘录如下。
1. 实验部分。1. 3 实验。无具体数据。
2. 结果与讨论。2.1 到 2.4 有数据,有表。资料太多,不再一一抄录,请看原期刊,
加注:
1. 为什么英国人,美国人能将部分德文资料译成英文,供大家参考?但不提供说明,是什么原因?
2. 我上面所列已公开德文原件,有1938年,1940年的,为什么国内无人去看?你知道英国人,美国人不会译错?是老的资料,应该看看吧。
陈忠源 2016年12月30日 祝大家新年好! 于 无锡 明辉国际。













