C.I. 冰染重氮组分2(C.I. 37005)CAS号 [108-42-9]
CAS名: Benzenamine, 3-chloro-, 发明者: Winther, Laska, Zitscher 1911年。
用途: 酸性红83, 110。分散橙51。分散红221。媒介黄16。溶剂红151。还原紫19。颜料橙4, 24。颜料红65。农药,医药中间体。
参考文献: Beil.12. 602; E1, 300; E2, 319; E3, 1303; E4, 1137. 生产工艺文献: FIAT 764 – Echtorange GC Base;
Echtorangesalz GC.
BIOS 986, 66-68.(=胶卷PB 77764)No. 27. m-Chloroaniline.
No. 28. m-Chloroaniline hydrochloride. I.G. Griesheim. 抄录如下。
反应式: 本人有加注,译者未说明译自哪个德文资料。
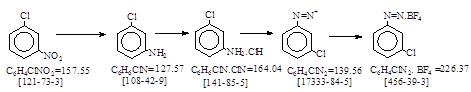
Iron reduction type
1, details as for o-Chloroaniline (No. 25)
Yield data and material consumption/tone
m-chloroaniline: m-Chloronitrobenzene 1.3 tone, Yield = 95% theory.
Service consumption/tone m-chloroaniline:
Electricity 377 K.W.H. Steam
8.8 t. L.P. /2.1 t. H.P. water 125 m3. Air 314 m3.
Plant for 40 tons/month as for o-chloroaniline:
1. 1
reducer 10 m3 brick-lined. 2. 2 settling cylinders, each 10
m3. 3. 1 still, 10 m3.
Analytical data:
m-chloronitrobenzene C.Pt. 43.50C. m-chloroaniline C.Pt. -100C. to -110C.
Annual production: 略。Reducer time cycle: 略。以下是No. 28. 生产工艺抄录。
600 kg. m-chloroaniline (No.27) is charged to 2000 l.
8% hydrochloric acid at 700C. agitated for a further 2 hr. after
completion of amine addition 500 kg. hydrochloric acid 240Be’ is
then added to precipitate the hydrochloride. Cool to 15-200C.
Isolate the hydrochloride on a vacuum filter and centrifuge to reduce the water
content. Dry 70-750C. and grind. The mother liquor is rejected after
6-7 batches.
Materials consumption/tone m-chloroaniline
hydrochloride: m-chloroaniline 0.865 t. Yield = 90% theory.
Analytical data:
m-chloroaniline C.Pt. -10 to -110C. m-chloroaniline hydrochloride 98.5% - 99%.
日文译文。 细田豊 《理论制造染料化学》 1957年。P. 458. 译自PB 77764. 抄录如下。
m-クロルアニリン: 煮沸水2 m3 に铁粉1.5 t,義酸15 kgを入れ,m-クロルニトヘ”ンセ”ン4100 kgを徐加し初め约1 tを加えた顷铁粉1.5 tと義酸15 kgを追加しまた後に铁粉1 tを追加しニトロの装入は17-20 hて”终る。还原终れは” m-クロルアニリン层を分离し,残りは水蒸汽蒸馏しともに真空蒸馏によつて精制する。还原收率95%。
m-クロルアニリン盐酸盐(フアストオレンシ”GCヘ”-ス): m-クロルアニリン600 kgを8% 盐酸2 m3に700て”加え2 h搅拌,240Be’盐酸500
kgを加え15-200に冷して真空滤過後远心分离机にかけ70-750て”亁燥する。收率90%。
中文译文。张澍声《精细化工中间体工业生产技术》1996年。P, 76. 译自BIOS 986. 抄录如下。
间氯苯胺和邻氯苯胺一样,采用铁粉还原。每吨间氯苯胺消耗间硝基氯苯1300 kg,为理论量的95%。凝固点 -10 – 110C.
600 kg间氯苯胺在700C 加到2000 L 8% 盐酸中,加完胺后再搅拌2小时,然后再加入500 kg 40% 盐酸以沉淀盐酸盐。冷却到15-200C,真空抽滤分离出盐酸盐,离心脱水降低水含量,70-750C干燥并研磨。母液重复使用6-7次后弃去。间氯苯胺盐酸盐纯度98.5 – 99%。
每吨间氯苯胺盐酸盐消耗间氯苯胺0.865 吨。间氯苯胺盐酸盐即橙色基GC,是冰染染料的重氮组分。
PB 25625, 304-307. Directions for producing the zinc chloride
salt of “Echtorange G Base.” 1937年10月。美国人介绍如下。
This product is obtained by diazotization of
m-chloroaniline and transformation into the zinc chloride double salt. 1美元,未抄录。
PB 25625, 308-315. Production of “Echtorangesalz GC” 1938年3月。 1美元,未抄录。美国人介绍如下。
The product is prepared by coupling naphthaline disulfonic acid with the
diazonium compound of m-chloroaniline. A complete description of the method is
given, including further processing and tests. In German.
PB 25625, 316-325. Production of “Echtorangesalz GC neu.” 1932年3月。 1美元,未抄录。美国人介绍如下。
This product is the borofluoride of the diazonium compound of m-chloroaniline. A
complete description of the process, using borax
and hydrofluoric acid, is given, In
German. 本产品CAS号[456-39-3].
PB 70422, 1997-1999. Fast Orange Salt GC new By
Prosiegel 1937年 12月30日。1.5美元。德文。未抄录。
PB 70422, 2000-2001. Fast
Orange Salt GC. By Prosiegel 1937年12月13日。 1.5美元。 德文。未抄录。
PB 74239, 123. m-Chloroaniline
hydrochloride. 1940年。 德文。未抄录。
PB 74386, 12-15. Sodium diazotate of
m-chloroaniline. 1940年。 德文。未抄录。
BIOS / DOCS /1156/1121/B22. Fast Orange GC Salt. 英国人译自德文。 [456-39-3] 未说明原件号。抄录如下。
Sulphuric acid 600Be’ (148 kg) is run into
cold water (1390 kg) in an enameled iron vessel, and m-chloroaniline (100 kg) is added in a thin steam, with agitation,
during 11/2 hours. The mixture is stirring for 3 hours
and cooled to below 00C by
circulation of brine. Diazotisation is
carried out by addition of a solution of sodium
nitrite (56.8 kg, ii.e. 5% excess) in water (130 l) during about 1/2 hour, keeping the
temperature between 00C and 100C. The usual tests are
carried out to ensure the presence of excess acid and a final excess of nitrous
acid, which should persist for 1/2 hour. The final
temperature should be 100C.
Active carbon (1.3 kg) is added, and after 1/2 hour’s
stirring the solution is filtered. Pure
borax (45 kg) is added to the filtrate and stirring is continued for 1/2 hour. The temperature is then reduced to 00C and hydrofluoric
acid (37.7 kg at 100%) is added as a solution of about 70%. The temperature
rises and again reduced to 00C. Further charges of borax (45 kg) and hydrofluoric acid (37.6 kg at 100%) are then added. The
hydrofluoric acid is drawn in under pressure from an iron container using a
lead inlet pipe passing below the surface of the liquid. Agitation is continued
at 00C for 6 hours and the precipitated diazonium salt is then filtered off and centrifuged. Yield of paste 167 kg at 51.8% (molec. Mass
127.5), i. e.86.5% of the theoretical. The product is mixed with anhydrous zinc
sulphate (87 kg) and dried at 40-450C. It is standardized to a
content of zinc sulphate monohydrate of 1.50 parts per part of 100% diazonium
salt (molec. Mass 127.5) and a strength of 15.1% (molec. Mass 127.5), the final
diluents being disodium naphthalene-1,6-disulphonate, which also serves as solubilising agent.
以上抄自 K. H. Saunders & R. L. M. Allen <Aromatic
Diazo Compounds> Third Edition. 1985年。P. 169-170.
上海染料工业汇编。 橙色基GC (间氯苯胺盐酸盐) 1976年。 P. 199. 抄录如下。
1. 还原: 还原釜中加入水850升,铁屑700公斤和硫酸亚铁70公斤,搅拌,升温至950C,于5小时内滴加间硝基氯苯600公斤,反应温度100-1020C. 加毕,在100-1020C 保温2小时。冷却至70-750C,加纯碱中和至pH = 7-7.5。将物料吸至蒸馏釜中进行水蒸汽蒸馏,直至无油状物馏出为止。馏出物分去水后吸至真空蒸馏釜内,在真空度650毫米汞柱以上先脱水,至内温达150-1600C 时收集精料。
2. 成盐: 成盐釜内加水290升及30%盐酸210公斤,然后在半小时内加入间氯苯胺200公斤。加毕,升温至65-700C,保温半小时。冷却至100C,放料抽滤,抽干。 总收率 90%。
国内研究动态:
袁俊盛(南化) 由硝基苯制造间氯苯胺(橙色基GC)新的技术路线研究。[J]化工技术资料, 1963,2, 30. 摘录如下。
硝基苯300克,用无水FeCl3 5.8克为催化剂,在40-450C通入干燥的氯气氯化至比重升至1.36-1.37/150C停止。氯化产物经水洗及纯碱中和后减压分馏,收集130-1450C/40 mm的馏份,约得315克粗制间硝基氯苯,再与22% NaHS溶液866克在搅拌下加热5小时,趁热分去水层。油层减压分馏,收集126-1350C/40
mm的馏份,约得158克间氯苯胺,产率50%。
胡拖平 陈宏博 (大工) 单氯代硝基苯加氢还原时脱氯规律的研究。 [J] 染料工业, 2000, 6, 11-12. 摘录如下。
将5 g氯代硝基苯,30 ml甲醇,0.7 g Raney – Ni催化剂,0.2 g助催化剂(CEN)加入100 ml高压釜中,在不同温度,压力等条件下加氢还原后,将催化剂分去。取样进行色谱分析。
HP6890气相色谱:色谱柱HP- 5玻璃毛细管柱30米;柱温900C; 程序升温到2000C,每分钟100C;载气氮气;氢火焰FID检测器。 表3 加助催化剂下,间氯苯胺的加氢结果:温度700C, 压力1.0 MPa, 间氯苯胺含量 89.9%, 苯胺含量8.2%,反应时间70分。其余,略。
闫茂文 柳 楠 陈宏博 (大工) Raney-Ni 催化加氢制备间氯苯胺。[J]染料与染色,2004, 5, 287-288(251). 摘录如下。
实验操作: 将10 g间硝基氯苯,加入带有电磁搅拌的200 ml高压釜中,再加入30 ml甲醇溶液和脱氯抑制剂CEN,在釜体密闭的条件下进行加氢还原,每隔15分钟关闭进气阀,测量反应釜内氢气的压力降,待釜体内压力不再降低,加氢还原结束。开釜过滤回收催化剂待下次加氢时套用,滤液常压蒸馏回收甲醇,再减压蒸馏得到间氯苯胺。
结果与讨论: 请见原刊物,本文已在美国化学文摘中有摘录,见CA. 143:327736.
加注:
PB 25625, 70422, 74239和PB 74386 胶卷国内均有收藏。
上面抄录的资料,除已有说明外,均未见有美国化学文摘的摘录。间氯苯胺重氮盐的其它成盐体暂时未査到其CAS号。可供参考的有对氯苯胺的重氮盐,如下:
Benzenediazonium, 4-chloro-, 1,5-naphthalenedisulfonate
(1:1)
CAS号 [77181-04-5] C6H4ClN2 .
C10H7O6S2 =
陈忠源 2017年3月13日 于 无锡 明辉国际。













