C.I. 冰染偶合组分5(C.I. 37610)生产工艺 CAS号 [91-96-3]
CAS名: Naphthalenecarboxamide, N,N’-(3,3’-dimethyl[1,1’-biphenyl]-4,4’-diyl)bis[3-oxo- 发明者:
Laska and Zitscher 1921年。
用途: 颜料黄16。颜料黄77。
生产工艺参考文献: FIAT 764 – Naphthol AS – G. 以下按本人手头资料整理如下:
BIOS 3597,1-7.(=胶卷 70421)Process for Manufacture of Naphthol AS –
G. (I.G. Offenbach) 英国人译自德文。抄录如下。
反应式: 本人有加注,译者未说明译自哪个德文原件。(2钠盐:CAS号 [68540-94-3])
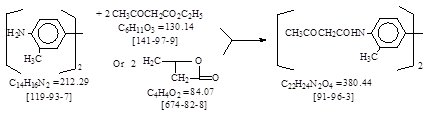
Mol. Wt: 380. Theoretical Yield: 179.2% of tolidine.
Scientific
Designation: 4,4’-di-(acetoacetyl)-3,3’-toluidine or 4,4’-di-(acetylacetamido)-3,3’-dimethyldiphenyl.
Equipment: 以下抄录不再分项。(其中有不少用的是德文,如Montejus
– 杨液器)
a) Enamelled charge kettle of 1500 liter
capacity, aluminumclad cover, steam jacket, aluminium column with “Raschig”
porcelain rings, distilling tube of lead, aluminium agitator with hollow. b) Cast-iron still of 2500 liter capacity,
with steam jacket, agitator with hollow shaft, distilling tube. c) Twin-column copper condenser for a). d) Condenser for b) (iron). e) Two separator vessels for c).
f) Two separator
vessels for d). g) Dissolving kettle
for tolidine, enameled, with steam jacket, vent pipe, hand stirrer, capacity
350 liters. h) Measuring vessel for
solvent naphtha, 1100 liters. i)
Measuring vessel for solvent naphtha, 500 liters. k) Montejus for solvent naphtha, 1000
liters. l) Feed kettle, 350 liter
capacity, with heating coil and inspection glass for tolidine. m) Round suction filter of wood, capacity
750 liters. n) Suction strainer vat of
2000 liter capacity with adjustable wooden stirrer. o) Intermediate vessel for solvent naphtha,
500 liters. p) Receiver, 12000 liters. q) Montejus, 1200 liters. r) Suction montejus, 3000 liters. s) Vacuum pump. t) Clay vessel for acetoacetic ester, 250
liters. u) Two clay touries for solvent
naphtha vapors. v) Cast-iron stirring
kettles, 1100 liters. w) Conical
separating kettle, 2000 liters, with wooden stirrer. x) Four montejus for alcohol, total
capacity: 10,800 liters. y) Rectifying
kettle for alcohol with column and condenser, 800 liters. z) Drying oven. Grinding drum.
Procedure:
750 kg of solvent naphtha (start of boiling at 1350C)
are pre-charged into the charge kettle, and traces of moisture are removed by
distilling-off about 30 liters (through column). Meanwhile the tolidine is
dissolved. In the dissolving kettle, 50 kg. of tolidine eff. (distilled in vacuo)
are first dissolved with 200 kg. of solvent naphtha at 1200C, while
operating the hand stirrer, and are forced into the upper feed kettle. 160 kg.
of acetoacetic ether are run into the charge kettle, and immediately afterwards
the feeding of the tolidine solution is begun. Temperature inside kettle:
135-1360C. Temperature on dephlegmator: 118-1200C,
cooling with or without cooling water, or with a little cooling water,
depending on time of year. The introduction lasts about 2 hours. Immediately
after pressing-up 50 kg. of tolidine eff. are once again dissolved as above,
and the solution is likewise run in during about 2 hours. Thus, the total
duration is approximately 3 1/2 hours. Steam: 4 – 5 atm. After-stirring and heating
at 1350C for 1 hour and 15 minutes. The feed kettle is after-rinsed
with 30 liters of solvent naphtha.
During the condensation 240 liters of solvent naphtha
including split-off alcohol and some acetone are distilled into the stirring
kettle, where they are stirred out with 130 liters of water. The entire
material is pressed into the conical separating kettle; the alcohol is
separated therein and stored in the alcohol montejus.
The solvent naphtha is stirred out with water two more
times, and the water is drained. This cleansing of the solvent naphtha is
important, since otherwise acetone and alcohol residues get into the charge and
interfere with it.
Processing of As – G:
The condensation mass is cooled to 200C and
blown into the suction strainer vat. The
solvent naphtha mother liquor is sucked off, and the AS-G is washed with 200 kg of solvent naphtha and subsequently with
200 liters of water, pasted up with water, and drained into the charge kettle.
This is filled up to 600 liters with water, and the adhering residues of
solvent naphtha are distilled off through the lead pipe and condenser, taking
care to avoid foaming.
At 900C the AS-G-mass is mixed with 5 kg of hydrochloric acid 200Be’,
stirred for half an hour, pressed onto the round suction filter, and thoroughly
washed with hot water.
The AS-G is
pounded well and dried on aluminum sheets in drying ovens at 1000C,
and is then ground. The enameled kettle is thoroughly cleaned with some soda
and water, and dried.
Yield: 172.3 kg of 98.5% = 169.7
kg pure, from 100 kg of tolidine with a content of 99%, foe example.
169.7 / x = 99 / x x = 171.4 pure,
instead of 179.2 = 95.6% of theoretical value.
Analysis:
1.9 g are dissolved in 130 cc of pyridine at 400C
(do not heat above this temperature). After cooling to about 50C, tenth-normal
aceto0-phenylenediamine diazonium chloride solution is slowly run in until an
excess of diazo solution can be demonstrated with H-salt in the outflow of a
drop of the solution on filtering paper for 3 minutes.
The number of cc consumed indicates the content in
percent. The temperature must not rise above 100C.
Test of Solubility:
50 cc of hot water and 5 cc of caustic soda solution
33% are poured over 5 g of AS-G. Temperature: 500C.
The specimen should be readily soluble.
Dyeworks: Dyeing and solubility
should be certified.
Regeneration of Solvent Naphtha Mother Liquor:
The liquor is stirred with 50 kg of caustic soda
solution 380Be’ at 700C for 1 hour; subsequently, the
solvent naphtha is blown off with live and dry steam. The first 300 kg of
naphtha are collected in a special vessel and stirred out in the stirring
kettle with 300 liters of water, and then precipitated. The balance of the
naphtha runs into the collecting montejus immediately after disyillation.
Alcohol Rectification:
One charge of Naphthol
AS-G from 100 kg of tolidine yields about 170 kg of dilute alcohol of
approximately 25% by weight; its concentration is increased to 90-91% by
rectification. 600 kg = 710 liters of spirit water are mixed with 15 kg of
caustic soda solution 380Be’ and distilled, splitting off only a
small after-run of about 16 kg, which is added to the next distillation.
The spirit thus obtained shows a strength of about 91%
in a hydrometer test. The acetone
content (about 5-6%) is determined by titration with iodine, so that the spirit
has a content of 85-86%.
The course of
the distillation of solvent naphtha is indicated below :
134.60 = 5 drops. 136.60 = 5 cc. 137.00 = 7 cc. 138.00 = 15 cc. 139.00 = 23 cc. 140.00 = 38 cc. 141.00 = 50 cc. 142.00 =62 cc. 143.00 = 71 cc. 1440 = 77 cc. 1450 = 82 cc. 1460 = 86 cc. 1470 = 88 cc. 1480 = 90 cc. 1490 = 91 cc. 1500 = 92 cc. 1510 = 93 cc. 1530 = 94 cc.
If after a number of charges the boiling point of the
naphtha in the charge kettle rises above 1360C, some low-boiling
solvent naphtha must be admixed. The
latter exhibits the following boiling behavior:
1310 = 5 drops. 1320 = 5 cc. 124.40 = 35
cc.
1360 =
70 cc. 1380 = 90 cc. 1390 = 75 cc.
It may be necessary to use high-boiling naphtha, too : 1300 = 5 drops. 1340 = 5 cc. 1420 = 35 cc. 1520 = 70 cc.
1690 =
90 cc. 1720 = 95 cc.
It is
favorable to the AS-G-yield to wash
all the Naphths-w thoroughly with water after a certain period of time.
Direction for Determining the Concentration of Naphthol
AS-G: mol. Wt. 380.
1.9 gm are dissolved in 150 cc of pyridine at 400C
(do not heat to higher temperature). After cooling to 5-100C,
tenth-normal aceto-p-phenylenediamine diazonium chloride solution is slowly run in, until an excess of diazo solution can be demonstrated with H-salt for 3
minutes in the outflow of a drop on filtering paper. The number of cc consumed
indicates the content in percent.
本书,当时《中外科学书社》和《The New China Book Company》均有影印,售价为:5,000元。
本资料目前未见到有中文译文。
PB 25625, 619-624.
Method for producing “Naphthol AS-G.
1938年2月。 德文生产工艺。 1美元。 美国人介绍如下。
This substance is N,N’-di-(acetoacetyl)-o-tolidine. A
description of the apparatus used is given. Tolidine is dissolved in solvent
naphtha and added to acetoacetic ester. The temperature is kept at 1350C. The process is
described, including further processing, analysis, and recovery of solvent
naphtha. The yield is 94.9% of the theoretical. In
German.
本缩微胶卷上海图书馆有收藏,其编号:F – 108. 当时美国报价:缩微胶卷6美元。放大本:117.5美元。
细田豊。《理论制造染料化学》 1957年。P. 645. 译自PB 25625. 未说明页号。抄录如下。
ナフト- ル AS-G: 1.5 m3 ホ-ロ-引釜ルヘントナフタ750 kg,トリシ”(真空蒸馏品)50 kg + ンソルヘ”ントナフタ240 kgの溶液(1200)を入れ,アセト酢酸エステル150 kgを加え,130-1360て”2
h後,トリシ”ン50 kg + ナフタ200 kgを追加,1350て” 3-4 h 反应し,その间エタノ-ルとソルヘ”ントナフタ240 lを留出する。200に冷却して滤過,ナフタ200 lて”洗い,水200 lて”洗つた後水蒸汽蒸馏し,盐酸5 kgを加え700て” 1/2 h搅拌後滤過水洗する。收率 94.9%。
PB 70061, 1616. Naphthol
AS-G 产品标准。未抄录。
PB 70361, 6966-6968. Naphthol
AS – G. (diacetoacetyl-o-tolidide). By Mengel. 德文生产工艺。1941年3月27日。未抄录。
本资料美国售价1.5美元。国内有收藏,美国售价:6美元。放大本:95美元。日本人未引用。
纳夫妥 AS-G 试制报告。[J]有机化学工业技术报导, 1959, 1, 63. (摘自上海化工研究院有关资料) 抄录如下。
1) 溶解: 将50 g联甲苯胺首先加入三口瓶中,继加溶剂油325 g加热至1400C,保持温度1小时使全溶。
2) 缩合: 250 g 溶剂油首先加入瓶内加热至1350C,蒸出其中水分,加80 g乙酰乙酸乙酯,保持135-1360C, 在135-1360C滴入联甲苯胺溶液约31/2时加完。维持1350C 75分钟。然后冷至200C, 停止搅拌。
3) 过滤:
4) 清除溶剂油: 水蒸汽发生瓶内装水600 cc,加热沸腾通出水蒸汽,先置入上述AS-G滤饼加水400 cc调成糊状直接加热。通入水蒸汽,全部蒸出溶剂油,然后冷却至900C加HCl (200Be’)2 1/2 g,搅拌30分钟。试验结果收率为86.25%。
加注:
历史回顾: 请见C.I. 冰染偶合组分2. 再请看《书评 -4 (英文版,染料索引 – 结构篇)》
几点想法:
完成《中国染料100年》一书的出版发行工作,这是《中国染料工业协会》提出,要在2017年完成的目标,我想可以通过上网的一些资料使大家了解过去,了解我国染料行业的起步和发展过程。在这里希望通过读者,提请中国染料协会能接纳我这位义工,因为我在上海染料展上已给过名片,未得到联系,可能考虑到我已老了!我的理念是以上网为养老,高兴过好每一天,不是我的同龄人,杨锦宗院士早已远离我们了!生命在于运动!我没有写书,但有用的东西不要带走!要留给后人。再次谢谢公司,说点击数(读者阅读数)到二万,庆祝一下,谢谢!我想应该到20万吧!200万吧!这里不应该是数字,而应该是对染料行业真正有用,对年轻人有用,能使他们学到东西,才是我的努力目标。因为我是国家培养的第一代大学生。而且是享受工资待遇的一代大学生。
在这里要谢谢沈阳院何岩彬同志,是他个人邀我参加了即将出版的《染料品种大全》的增补工作,当然我是免费的,如果我上网的资料还有用,就请大家订阅吧!如有问题可以找我,因为到目前活得还可以!谢谢!!!
陈忠源 80后义工 2017年6月7日 于 无锡 明辉国际。













