C.I. 冰染重氮组分33(C.I. 37075)生产工艺。 CAS号 [121-27-7]
CAS名:Benzenamine, 5-chloro-2-(4-chlorophenoxy)- 历史参考文献:Beil. 待检索。
发明者:Montmollin, Bonhote, Spieler 1922年。生产工艺参考文献:FIAT 764 – Echtrot FR Base; Echtrotsalz FR.(原版Colour Index).
用途:有机合成,织物染色。LookChem网登录生产与经营单位:色基51家;盐酸盐2家;重氮体和稳定重氮盐各1家。
BIOS 1149, 37-40.(=胶卷PB 80376)。 Fast Red FR Base.(4,4’-Dichloro-2-aminodiphenylether)。英国人译自德文,无资料来源。
反应式:本人有加注,暂未找到德文原件,其成盐体的工艺未抄录。CAS号[125-12-6],已在2017年11月16日上网。
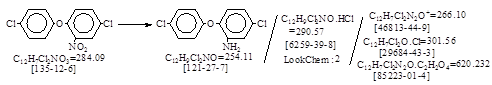
Plant: 设备:2000 l. reduction pan。 3000 l. C.I. extractor。 3000
l. wrought iron still。 3000 l. C.I. settling vessel。
Process:操作步骤:
The reducer is charged with 200 l. of water, 100 kg. of iron borings
and 30 kg. of formic acid, and heated to the boil. The molten 4,4’-dichloro-2-amino-diphenyl
ether (600 kg.) is run in during 3 hours with simultaneous addition of 250 kg.
of iron. Heating by jacket steam is
continued until reduction is complete – 16 hours total time including charging.
The reduction mass is cooled, allowed to settle to separate water and
oil blown to the extractor (see Notes).
The extractor is charged with 1500 l. of benzene, the solvent separated
and blown to the still; this is repeated 8 times in all using a total of 12000
l. of benzene.
The benzene is distilled off in 5 or 6 lots and the residues
combined. Vacuum is applied and any
chlorophenol remaining from the crude nitro-compound then distilled. The chlorophenol-free batch is then blown to
the final still where several batches are distilled together in vacuo. The product boiling at 245 – 2600 at 25 mm.
is collected. Yield: 93% theory on
nitro-compound.
Analysis: C. Pt.= 61℃. (pure 61.3℃). Costa : Materials Rm.
150. Expenses Rm.
20.
Notes: Difficulties were experienced with extraction due to
emulsification. In the process as written,
water was separated prior to solvent extraction but this was not very
satisfactory. Addition of Nekal, Monopol
brilliantoel, Igepon or Humectol facilitated the separation.
In 1940 a different technique was tried out: the extractor was charged
with 2000 l. of benzene and only 200 l. of the iron reduction mass was blown
into this. After separation of the
solvent, a further 1400 l. of benzene was added, and, after agitation, again
separated. Then 1200 l. of benzene was
added followed by a further 200 l. of the iron reduction mass. This system of extractions was repeated until
all the reduction mass had been treated.
Fast Red FR Base. (4,4’-Dichloro-2-aminodiphenyl ether hydrochloride).
Plant: 3500 l. agitated vat,
tiled with Hoechst tiles and cement。 Lead
stirrer, lead piping, and acid-resisting tiled nutsches。
Process: 操作步骤:
The vat is charged with 1000 l. of condenser water and 1000 kg. of
crude hydrochloric acid 19.50Be’ and heated to 400. The molten (70℃) 4,4’-dichloro-2-aminodiphenyl ether (500 kg.) is run in during 2 – 3
hours, the air in the vessel being replaced by nitrogen to minimize
oxidation. The temperature is held at 700
during the addition and is then cooled during 12 hours to 15 – 20℃. The precipitated
hydrochloride is blown to the nutsche, pulled dry and washed three times with
saturated brine (app. 500 l. each).
The washed hydrochloride is dried in vac. at 40 – 500 in the central
drying department, ground and sieved through 40 mesh. The dry product is usually 90% as
hydrochloride (120 – 125 wt. % of the base charged) and is standardized to 85 –
86% with salt. Yield = 95 – 98.5%
theory.
张澍声《精细化工中间体工业生产技术》。《染料工业》编辑部出版。1996年。P. 22.
4,4’-二氯-2-氨基二苯醚盐酸盐,译自BIOS 1149, 37。(抄注:译者未说明是:C.I. 冰染重氮组分33.)
4,4’-二氯-2-氨基二苯醚:
在2000 L还原锅中加入200 L水,100 kg铁屑和30 kg甲酸,加热至沸。于3小时内加入600 kg熔融的4,4’-二氯-2-硝基二苯醚,同时加入250 kg铁屑。加热至还原完成,包括加料总时间16小时。
还原物冷却,静置,分离出水,油层送入萃取器。萃取器中加入1500 L苯,溶剂分离出来送入蒸馏釜,重复8次,总计用苯1200 L。蒸馏出苯,5或6批的馏余物合并。抽真空,先蒸馏出留在硝基物中的任何氯代苯酚。不含氯代苯酚的反应物压往另一蒸馏釜中,收集245 – 260℃ /25 mm的产品,结晶温度61℃(纯品61.3℃)。以硝基化合物计,收率93%。
4,4’-二氯-2-氨基二苯醚盐酸盐:
在3500 L锅中加入1000 L冷凝水和1000 kg 30% 盐酸,加热到40℃。于2 – 3小时内流入500 kg 70℃的4,4’-二氯-2-氨基二苯醚,锅内空气用氮气代替减少氧化,加料时保温保持70℃,然后于12小时内冷至15 – 20℃。沉淀的盐酸盐抽滤,用饱和食盐水洗3次,每次约500 L。在40 – 50℃真空干燥,研磨,过40目筛。收率95 – 98.5%。
干产品为90% 4,4’-二氯-2-氨基二苯醚盐酸盐,用食盐标准化至85 – 86%,即为Fast FR Base.
PB 70422, 2019-2020. Fast Red
Salt FR K. By Prosiegel. 稳定重氮盐德文生产工艺,1941年9月26日。1.5美元,未抄录。
PB 70422, 2021-2022. Fast Red
Salt FR new. By prosiegel. 稳定重氮盐德文生产工艺。1938年2月23日。1.5美元,未抄录。
【抄注】因为未抄录原件,所以未知成盐的组分,也无法知道成盐体的CAS号。
PB 82233, 250-251. “Echtrotbase FR”. 1946年德文生产工艺。2美元,美国人介绍如下。
2-Amino-4,4’-dichlorodiphenyl ether hydrochloride. 即CAS号 [6259-39-8]。未抄录。
何岩彬 主编《染料品种大全》。沈阳出版社 出版。2018年。P. 401-402.
C.I. 冰染重氮组分33。
【参考文献】缺BIOS 1149,37-40。其中PB报告为本人所提供,当然这已是过时的历史资料!抄录的目的:有这样的资料未被利用!
另外,有关CAS号,本人已列入CAS号编目中,可供读者参考!当然,目前不可能上网!因为本人的工作还在进行中。
陈忠源 2020年6月29日星期一。













