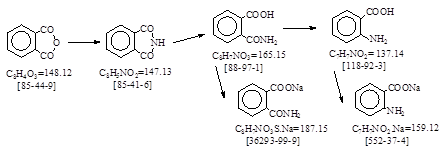
CAS号 [118-92-3] 生产工艺 邻氨基苯甲酸
CAS名: Benzoic acid, 2-amino- 参考文献: Beil. 14, 310; E1, 529; E2, 205; E3, 879; E4, 1004.
用途: 酸性黄59, 121, 241, 259。
酸性红2, 442。
酸性绿43, 125。
酸性棕425。
直接黄39, 130。
直接橙94。
直接红99, 186, 250。
直接棕112。
分散黄32, 77, 79。
分散红364。分散蓝181。 媒介黄8, 57。
媒介红9, 32, 60, 74。
媒介紫2。媒介棕40。媒介黑106。颜料黄142,151。颜料红50,50:1,50:2,60,100,20。活性棕7,8,9,10.
医药及其他有机合成等。
生产工艺文献:按本人手头资料整理如下:(至于有否新的专利,本人已不可能再去査CA了!)
BIOS 986, 53-55.(=胶卷PB 77764)Anthranilc acid (Anthranilsaeure) (I.G.
Ludwigshafen). 英国人译自德文。抄录如下。
反应式: 本人有加注,译者未说明译自哪个德文原件。(以下抄录文,仅供無此类资料的朋友参考!可能有抄错的地方。请谅解!)
Plant Description:以下抄录不再分项。
(52) 1 x 1 cu.m.
M.S. measure vessel on weighing machine.
(700A&B) 2 x 3 cu.m. C.I.
agitated kettles with spade agitators at 65 r.p.m. and with ammonias cooling. (101)
1 x 3 cu.m. C.I. agitated kettle, spade agitator at 40 r.p.m.
jacketed. (102) 1 x 3 cu.m. agitated C.I. kettle with Finger
agitator at 40 r.p.m. and ammonia cooling.
(113) One M.S. rubber-lined
kettle with Balken agitator at 22 r.p.m.
(106A) One M.S. Kerasolithiert
and stone-lined nutsche, 6 cu.m. 8 sq.m.
(112) 1 x 10 cu.m. M.S.
rubber-lined pressure vessel. (106) One M.S. Korasilithiert and stone-lined
nutsche, 6 cu.m. and 8 sq.m. (111) 1 x 7.5 cu.m. M.S. rubber-lined pressure
vessel.
Process Description:(1) Preparation of Chlorine Solution(This takes 17 hours). 抄注: NaOCl=75.45. CAS号[7681-52-9].
Into each of the two kettles 700 A and B run in from
measure vessel (52) accurately 1,300 caustic soda 50% and follow this with
1,835 kg. drinking water running the water through the measure vessel. Cool to
+ 20C. and at 170C. begin the addition of chlorine. In 16 hours add to both
kettle together at 00 to + 50C. 576-580 kg. chlorine gas,
that is 18-18.1 kg. per kettle per hour. Estimate the chlorine content by N AS2O3 and cool
to – 80C.
(2) Preparation of
Phtalaminsaeure Natrium (This takes 5 hrs.). 抄注: CAS号[36293-99-9].
Into kettle (101) add from measure vessel (52) one
after another 840 kg. drinking water, 138 kg. ammonia 100% as 25-27% ammonia
solution and 10-20 l. drinking water. Charge 602 kg. phthalic anhydride ground
fine from casks during 3 min. The temperature rises from 170C. to
60-620C. The pH on Merck’schem
paper = 8-7.5. Cool during 30 hr. to 38-400C. A small amount of
ammonium phthalate crystallizes. Empty the jacket and charged 602 kg. phthalic
anhydride in 9 min. Follow 20 secs. Later with 650 kg. caustic soda from
measure vessel (52) and 290 l. water in a total of 14 min, The temperature
rises to 63-650C. 5 min. after the charging is finished there is a
clear only faintly yellow solution of pH 8-7.5. Cool to 36-400C.
during 1 hr. Immediately drop the contents of (101) into (102) and wash
through with about 25 l. Rhine water. In (102) cool at once with ammonia to -120C.
(2-21/2 hr.).
(3) Oxidation:
Immediately kettle (102) (700A & B) are cooled to
the specified temperatures stop the agitators and drop kettle (102) in 130 sec.
into (113) and immediately wash through with about 100 l. drinking water. Start
the agitator of (113) and run in the chlorine
liquor simultaneously from (700A & B) in 110 sec. The temperature in a
normal batch runs as follows:-
Time Temperature
(From start of
chlorine solution addition) In not
more than 5-51/2 min. the normal temperature of 250C.
is reached.
1 min. 50 sec. + 1 to + 30C. The temperature rises in about 50 sec. to
60-650C. and in a further 5 min.
3 -“- + 6 to + 80C. to about 720C. Stir 1/2- 3/4 hr. longer and test: 50 c.c. should
require
4 -“- + 9 to + 110C. 44-46 c.c. N sodium nitrite 9.9 – 11 wt% anthranilsaeure, i. E.
5 -“- + 18 to + 210C. 95-99% theory.
(4) Isolation:
During about 2 hr. run in 1,300 kg. sulphuric acid
adding 30 kg. sodium bisulphite and 20 kg. carboraffin 1200 kg. sulphuric acid
has been added. Finally the liquor must be faintly acid to litmus (pH = 6.5)
and Brilliant Yellow paper still shows faintly. During the addition of the last
100 kg.sulphuric acid 500 the lid, walls and agitator are washed
down with 500 l. drinking water. The temperature is now about 640C.
Cool with the “Kuhlohr” to 52-530C. (560C in winter) in
4hr., and filter off the carbon on nutsche (106A) dressed with two cotton
cloths the filtrate passing to (112). The filtrate must be clear and free from
carbon. The empty vessel is immediately washed with water, only the very first
wash water going to the nutsche, the rest to the drain. The wash water must
finally have no reaction upon litmus. Transfer the batch from (112) to (113)
and wash the nutsche cake with 400 l. water. The wash-liquor goes to (113). The
carbon residues containing over 3 kg. anthranilic acid by test are collected and
the anthranilic acid dissolved out later with a little caustic soda. The
anthranilic acid solution in (113) is now about 38-400C. Run in 140
kg. hydrochloric acid 30% during 4 hr. and seed with material from a previous
batch. The anthranilic acid must precipitate in glistening small plates. If it
precipitates in flocculent needles allow to stand, and quite slowly add the
acid to settled batch. After the hydrochloric acid run in during 5-7 hr. 450
kg. sulphuric acid 500. The final temperature is 400C.
dark Congo paper shows grey-black, and the pH is about 4. If too acid phthalic
acid is precipitated. A sample of the filtered anthranilic acid must be clearly
soluble in dilute hydrochloric acid. Filter on nutsche (111) and cover with
1,300-1,500 l. drinking water. The last wash-liquor has a density of 0.50Be’,
the mother liquor 23.5-24.50Be’. Both the mother liquor and wash
liquor are discarded. The nutsche cake weights 1,050-1,250 kg. and is dried in
Schildelufttrockenschrank at 55-600C. in about 20 hr.
Yield: 980 kg. per batch of about 99.5% dry
content. Estimated by titration with N/1 caustic soda according to I.G. Analyse
Nr. 335.
= 88% theory from phthalic anhydride.
Quality: 99.1-99.5% (I.G. Analyse Nr. 335). M.P. 1450C. Clearly soluble in
hydrochloric acid and caustic soda. Density 50-60 kg/100 c.c.
细田豊。 技報堂出版。《理论制造染料化学》1957年。 P.520. 译自PB
77764. アントラニル酸。抄录如下。(1)フタルアミン酸ナトリウム: [36293-99-9]. 水850 l + 25% NH3水550
kgに無水フタル酸602 kgを加えれは”60-620に上
る. 30 hて”38-400に冷し無水フタル酸602 kgを追加,NaOH 50%液650 kg,水290 lを14 mに加えれは”63-650に上り,pH 8-7.5
の淡黄色透明な液となる。1 hて”36-400に冷し,アンモニア冷却器て” -120に冷す。
(2)酸化: [118-92-3]. コ”ム张酸化机に前记溶液を移し,(NaOH 50%液1300 kg + 水1835 kg + Cl2 580 kg)のNaOCl液を约2m
て”加えれは”温度上昇して720に逹しなお30-45 m搅拌する。
(3)分离: 500Be’硫酸1.3
tを约2 hて”加え,その1.2 tを加えた顷NaHSO3 30
kg,脱色炭20 kgを加える。pH 6.5, 52-530
て”滤過し,滤液に盐酸140 kgを4 hに加え,500Be’硫酸450 kgを5-7 hに加え,400 pH 4て”アトラニル酸を滤過し水1.3-15 m3
て”洗い,55-600て”亁燥する。980
kg (纯度99.5%),收率88%。
张澍声。《染料工业》编辑部出版。《精细化工中间体工业生产技术》1996年。P. 48-49. 邻氨基苯甲酸。抄录如下。
(1) 氯水的制备(抄注:次氯酸钠的制备): 在3 m3槽中准确计量加入1300 L 50% 氢氧化钠溶液,随后加入1835 kg水。冷
却下通氯,在0-50C通入576-580 kg氯气,即每小时通氯18-18.1 kg,用0.1 N AS2O3测定氯含量,并冷却到-80C,有两个氯水槽同
时操作。
(2)邻氨甲酰苯甲酸钠的制备: 在3 m3的锅内加入840 kg水,138 kg 100% NH3制成的25-27%氨水和10-12 L水,于3分
钟内加入602 kg苯酐细粉。温度由170C升至60-620C,pH为7.5-8。在30小时内冷却到38-400C,少量的邻苯二甲酸铵结晶出来。
于9分钟内加入602 kg苯酐,20秒后加入650 kg氢氧化钠和290 L水,加水和氢氧化钠的总时间为14秒,温度上升到63-650C.
加料后6分钟产生清晰的浅黄色溶液,pH为8-7.5。于1小时内冷却到36-400C,转入另一槽中,于2-2.5小时内用液氨冷却到
-120C。
(3)氧化: 迅速冷却到指定温度后于130秒内转移到另一槽中,于110秒内在搅拌下加入氨水,其正常操作温度为:1分
钟50秒为1-30C;3分钟为6-80C;4分钟为9-110C; 5分钟为18-210C。在5-5.5分钟内正常温度达到250C,于50秒内上升到60-650C
再经5分钟上升到720C,再搅拌30-45分钟。检验: 50 ml反应液需44-46 ml 1 亚硝酸钠溶液,相当于9.9-11%(重量%)邻氨基
苯甲酸,即理论量的95-99%。
(4)分离: 于约2小时内加入1300 kg硫酸,当已加入1200 kg硫酸时同时加入30 kg亚硫酸氢钠,最后溶液必须对石蕊
为明显酸性,pH 6.5。当加入最后100 kg硫酸时,盖子,器壁和搅拌器用500 L水洗涤,这时的温度约640C。于4小时内冷却到
52-530C,过滤。滤液必须是清晰的,不含碳。碳渣含3 kg以上邻氨基苯甲酸,用少量氢氧化钠溶液溶解。
滤液为邻氨基苯甲酸溶液,温度约38-400C,于4小时内流入140 kg 30% 盐酸溶液,并加入上一批的晶种。邻氨基苯甲酸以
闪光小片状沉淀。如果以絮凝的针状结晶沉淀,则放置并极缓慢地加酸到静置液中。于5-7小时内流入盐酸后,加入450 kg硫
酸。最终温度为400C,深色刚果红试纸呈灰黑色,pH约为4。如果酸性过强则沉淀出邻氨基苯甲酸。过滤的邻氨基苯甲酸必须
清晰溶解于稀盐酸中。抽滤,并用1300-1500 L水覆盖,最后的洗涤液比重为1.004,母液1.189-1.200。母液和洗液均弃去。滤
饼重1050-1250 kg,在55-600C于空气干燥箱中干燥约20小时。
每批得到980 kg 99.5%干产品,以苯酐计收率88%。熔点1450C,含量99.1-99.5%。
PB 17692, 926-928. Anthranilic acid. 分析方法。 Nr. 335. 未抄录。
PB 25602, 465-474. No.2062-2. Anthranilic acid. Solid I. 德文生产工艺。沈阳院收藏编号:BP。32-42.未抄录。
PB 25623, 293-296. Anthranilic acid. (From Phthalimide ). 德文生产工艺。未抄录。
PB 70063,237-243. Anthranilic acid. 德文生产工艺。1938年2月12日。未抄录。
PB 70063, 244. Anthranilic acid. K-salt. 德文生产工艺。 1939年7月31日。未抄录。
PB 70063, 432-433. Anthranilic acid, Purified. 德文生产工艺。 1939年3月3日。未抄录。
PB 73616, 1428-1434. Anthranilic acid. 德文生产工艺。 1945年。未抄录。
俄 A.B. Eльцова。 染料及中间体实验室合成方法。 1985年。 §5.26. 还原翠兰 ZX。译文如下。
苯二甲酰亚胺: [85-41-6]. 将装有直径大于10 mm空气冷凝器的500 ml园底烧瓶(耐热),固定在三角架上,加入50 g苯酐
和44.5 ml 28%氨水溶液,瓶内物料通过煤气喷灯慢慢加热成为均匀,完全融化的物料(在约3000C,经过2-3小时),加热过程
中定期摇动烧瓶,并通过冷凝器用玻璃棒把升华物铲入瓶内,趁热把反应物料倒入100 ml瓷皿内,为避免升华损失,皿上盖上
滤纸,冷却后将苯二甲酰亚胺进行粉碎,一般无需另行精制。 得量:47
g(95%),微黄色粉末,熔点232-2350C。
邻氨基苯甲酸: [118-92-3]. 将装有搅拌,温度计和滴液漏斗的500 ml瓷杯,置于装有小电炉的冷却浴中,加入82 g 氢氧化
钾,108 ml水,搅拌10-15分钟,加入300 g碎冰,在冰盐浴(-150C)冷却下滴加9.6 ml 溴,加溴速度控制在料温不超过100C,
搅拌30分钟,然后加入30 g磨细的苯二甲酰亚胺,加入速度控制料温不高于00C,将制得的清晰液冷至-50C,加入30 g 磨细
(在瓷研钵内研磨)苛性钾,烧杯内物料再搅拌30分钟,为使反应完成,慢慢加热至700C,加入7.5 ml 36% 亚硫酸氢钠溶液,
使过量次溴酸分解,冷却,过滤,清晰的滤液用45-50 ml 浓盐酸中和,反应介质用广泛试纸测应为碱性,随后加入30-35 ml
冰醋酸使邻氨基苯甲酸析出,用布氏漏斗过滤,抽干,用15-20 ml 冰水洗涤,于60-650C干燥。
得量: 24 g (86%), 浅黄色粉末,熔点143-1450C,可溶于乙醇,无机酸,稀碱。
加注:
以上列出的PB报告,国内均有收藏,它仅仅是本人编目的一部分,是否有参考价值,请读者评述。
陈忠源 2017年5月31日 于 无锡 明辉国际。













