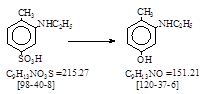
CAS号 [120-37-6] 生产工艺 3-(乙氨基)对甲酚
CAS名: Phenol, 3-(ethylamino)-4-methyl- 参考文献: Beil. (待补!)
用途: 酸性红50。碱性红1, 1:1。
溶剂红229, 237。颜料红81, 81:1 到81:6, 169。
生产工艺文献: 按手头资料整理如下:
BIOS 986, 196-197.(=胶卷PB 77764)No. 124.
2-Ethylamino-p-cresol (Aethylkresol
rein) (I.G. Ludwigshafen) 抄录如下。
反应式: 本人有加注,译者未说明译自哪个德文原件。其实是德文原件的摘译!
Process Description:
Charge 975 kg. potash 88-90% and 310 kg. caustic soda
50% solution to a C.I. melt kettle fitted with total condenser, and heat to 2300C.
At 230-2400C. charge 925 kg. aethyltoluidinsaeure 100% M.W. 215 (No.
128) in the form of 80-85% nutsche cake during 2 hr. Towards the end of the
addition charge carefully because of frothing. The kettle is closed except for
the connection to the condenser and heated for about 65 hours. In the first 24
hours heating hold at 240-2420C. During the next 20 hr. raise to
about 2460C. and then after this 44 hours take a sample and estimate
its sulphite content iodometrically – there is usually a 75-80% conversion at
this time. In the next 20-24 hours, misc the temperature to 250-2520C.
The melt is then finished. A sulphite estimation indicates 100% conversion.
Total time about 65 hours melting.
Dilute the finished melt in 1,000 l. water wash out the
melt kettle and dilute to 4 cu.m. water. Filter off the precipitated sulphite
and add 1,350 kg. hydrochloric acid 30% to the melt solution at 50-600C.
in a C.I. agitated pan until the liquor is only weakly alkaline to Brilliant
Yellow. Stir until cold, run the mother liquor to drain and wash the
precipitation ethylcresol once with 600 l. hot water, stir until cold, and
separate the washings for the next batch. Dry at 1300C.
Distil the dried raw cresol in a M.S. vacuum
distillation still. The pure product runs to the receiver at a still
temperature of 163-1900C and a vacuum of 12-8 mm. in the kettle and
9-6 mm. in the receiver. Run the melton distillate to a cooling pan.
Yield: 392 kg. pure ethylcresol
per batch = 42.4 kg.% from aethyltoluifinsaeure Mo. 215 =60.4% theory.
Quality: Setting point be not less than 870C.
张澍声。《精细化工中间体工业生产技术》1996年。P. 9. 3-乙胺基对甲苯酚。译自BIOS986, 196. 抄录如下。
在配有冷凝器的铸铁熔融锅中加入975 kg 88-90% 氢氧化钾和310 kg 50% 氢氧化钠溶液,加热到2300C。在230-2400C于2小时内加入925 kg 100% N-乙基邻甲苯胺-3-磺酸(以80-85%滤饼状态),在加料末期要仔细小心,因为发生泡沫。闭锅,但与冷凝器连接,并加热约65小时,在最初24小时,加热保持在240-2420C, 其次20小时上升到约2460C。经此44小时后,取样用碘量法测定亚硫酸钠含量,这时通常有75-80% 转化。第三阶段20-24小时升温到250-2520C。然后熔融结束,亚硫酸盐检测证明100% 转化。总时间约65小时。
用1000 L水稀释熔融物,并用水洗涤熔融锅,最后用水稀释到4000 L。过滤出沉淀的亚硫酸盐,在50-600C向熔融物溶液中加入1350 kg 30% 盐酸,直至溶液对亮黄试纸仅为弱碱性。搅拌冷却,将母液排放弃去,用热水600 L洗涤乙基氨基对甲苯酚一次,搅拌冷却,分离出的洗水用于下一锅反应。在1300C干燥。
在软钢真空蒸馏釜中蒸馏干燥的粗品N-乙胺基对甲苯酚,在163-1690C, 12-8 mmHg(锅内)收集纯的产品,接受器压力9-6 mmHg。
每批得到392
kg 纯的N-乙胺基对甲苯酚,熔点870C,收率60.4%。
PB 25602, 1103-1109. Aethylkresol rein. 3-(乙氨基)对甲酚。 德文生产工艺原件。未抄录。
PB 70063, 418-422. Manufacture of
2-ethylamino-4-hydroxy-1-methylbenzene. 德文生产工艺。1945年10月16日。未抄录。
陈忠源 2017年6月4日 于 无锡 明辉国际。













