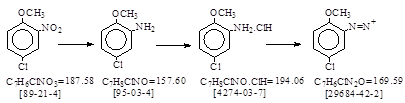
C.I. 冰染重氮组分10 (C.I. 37120)生产工艺 CAS号 [95-03-4]
CAS名: Benzenamine, 5-chloro-2-methoxy- 参考文献: Beil. 13, 383; E1, 318; E2, 183.
发明者: Winther, Laska, Zitscher 1912年。反应类别: 硝基还原。合成盐酸盐。重氮化和合成稳定重氮盐。
用途: 冰染偶合组分34, 51。 酸性紫56, 58。
酸性蓝154。
颜料红93。
溶剂紫2。
生产工艺文献: 原版Colour Index: BIOS 986, 69-72,
98-99. FIAT 764 – Echtrotsalz RC. 以下按本人收录的资料整理如下:
BIOS 986, 69-72.(=胶卷PB 77764)No. 30. 4-Chloro-o-anisidine
(crude). (I.G.
Griesheim) 英国人译自德文。抄录如下。
反应式: 本人有加注,译者未说明有资料原件!其实,有不少德文原件,今天应该是第一次公布,可惜未抄录!
Iron reduction of 4-chloro-o-nitroanisole (No.
52). Iron reduction type 3, with
solvent extraction of product.
4000 kg. 4-chloro-o-nitroanisole is charged to 1500 l.
water and 15 kg. Formic acid. The mixture is brought to the boil and 4000 kg.
iron borings added at such a rate as to maintain the desired rate of reaction.
15 kg. Formic acid is added when 2000 kg. iron borings has been added or when
the reaction tends to slow down. Time of addition of iron borings: 10-12 hr.
After completion of reduction 800 l. solvent naphtha is
added to obtain a better separation than is possible by steam distillation.
After a settling period of 1/4 hr. the naphtha layer is
drawn off to the agitated settling cylinders. The iron oxide is extracted a
further two or three times with 800 l. naphtha per extraction. The naphtha
extracts are combined after a further period of settling and filtered. Solvent
naphtha is removed by live steam distillation and the 4-chloro-o-anisidine
water mixture then worked up by vacuum distillation. On account of the
instability of 4-chloro-o-anisidine only small charges are distilled at a time.
Materials consumptions/tone of 4-chloro-o-anisidine: 4-Chloro-o-nitroanisidine 1.28
t. Yield
= 93% theory.
Iron borings 1.25 t. Formic acid 0.01 t. Naphtha 1.00 t. (recovery not given)
Services consumption: Reducer cycle time:
Electricity 820 K.W.H. Addition of nitro
body 10-12 hr.
Steam 20 t.L.P.
6 t. H.P. Steam
distillation 3-4 hr.
Water 150 m3. Air
635 m3. Total cycle time 27-33 hr.
Analytical data:
4-chloro-o-nitrianisole
M.Pt. 950C. 4-chloro-o-anisidine (Crude) M.Pt. 790C.
Plant required for 12 tones/month of all amines
requiring solvent extraction:
1. 1 x 10 M3 reducer brick-lined, ferro-silicon impeller agitator and reflux gear. 2. 1
agitated cyclinder used for settling 8 M3.
3. 1 storage vessel 10 M3. 4. 1
still 10 M3 for naphtha steam distillation, with naphtha-water
separator. 5. 1 vac. still 1.5 M3 for final
distillation of 4-chloro-o-anisidine.
BIOS 986, 71. No. 31.
4-Chloro-o-anisidine Pure. 抄录如下。
Crude 4-chloro-o-anisidine from the iron reduction
process contains approx. 4% impurities and these are removed by naphtha crystallization
and washing.
80 parts by weight 4-chloro-o-anisidine crude and 20
parts by weight solvent naphtha are heated to obtain solution and then cooled
slowly to 200C. in approx. 20 hr. The mother liquor is then run off
from the bottom of the crystalliser and then crystal mass washed with 10 parts
by weight of fresh naphtha. After draining off the washings the pure
4-chloro-o-anisidine is melted up and run into the still where naphtha is first
removed and the pure product then distilled over. The still charge should not
be greater than 500 kg. 4-chloro-o-anisidine due to the instability of the
latter. The naphtha mother liquors are discarded after recovery of naphtha by
steam distillation.
Materials consumptions/tone 4-chloro-o-anisidine pure: 4-chloro-o-anisidine 1.17
t. Yield
= 85.4% theory.
Naphtha 0.1
tones nett (i.e. overall yield 4-chloro-o-anisidne pure from 4-chloro-o-nitroanisole
= 79.5% theory).
Services consumption/tone 4-chloro-o-anisidine pure: Electricity 274 K.W.H.
Steam 6.0
t.L.P. 2.5 t.H.P. Water 100 M3. Air 155 M3.
Analytical data: 4-chloro-o-anisidine crude C.Pt. 78.50C. 4-chloro-o-anisidine pure C.Pt.
810C.
Plant: 1. 1 x 8 M3 crystalliser occupation
10 days/batch. 2. 1 x 0.8 M3 steam heated still for
distillation of pure product.
Annual production: 1937 59,000 kg. 1943.
BIOS 986, 72. No. 32. 4-chloro-o-anisidine Hydrochloride.(抄注:CAS号[4274-03-7])抄录如下。
600 kg. 4-chloro-o-anisidne crude is charged to 2000 l.
8% hydrochloric acid at 700C. The mixture is agitated for a further
2 hr. at 700C. 450 kg hydrochloric acid 240Be’ is then
added. The whole is then cooled to 15-200C. The hydrochloride is
filtered off on a vacuum filter, centrifuged to reduce the water content, dried
at 70-750C. and ground. The mother liquor is discarded after every
7-8 batches.
Materials consumptions/tone of 4—chloro-o-anisidine
hydrochloride: 4-chloro-o-anisidine 0.85 t. Yield = 95.7% theory.
Services consumptions/tone of 4-chloro-o-anisidne
hydrochloride: Electricity
272 K.W.H. Steam 12.2 t.L.P.
Water 105 M3. Air 40 M3.
Analytical data:
4-chloro-o-anisidine crude
C.Pt. not less than 78.50C.
Plant: For 20 tone per month of all
hydrochloride see plant for o-chloroaniline hydrochloride. (抄注: CAS号[137-04-2])
细田豊。 《理论制造染料化学》1957年。P. 493. 译自PB 77764. 抄录如下。
4-クロル-2-アニシシ”ン: ニトロ4 t, 水1.5 m3,義酸30 kg,铁粉4 t て”还原,ナフタ抽出,蒸馏等5-メトキシ-o-トルイシ”ンの场合と同样。粗制收率93%。
精 制: 粗制(约4% の不纯物を含む。 Cp 78.50)80部をナフタ20部と热して溶し,20 hて”200に冷し母液をぬき, ナフタ10 部て”洗い,蒸馏にかける(一度に装入500 kg以下)。收率85.4%,cp 810
4-クロル-o-アニシシ”ン盐酸盐 (Fast Red RC Base): 粗制600 kgを8% 盐酸2 m3と700て”2 h搅拌,240Be’ 盐酸450
kgを加え,15-200て”盐酸盐を滤過,セントルて”脱水,70-750て”亁燥する。收率95.7%。
张澍声 编译。《精细化工中间体工业生产技术》 1996年。P. 70. 译自BIOS
986,69-71. 抄录如下。
4-氯邻氨基苯甲醚(粗品): 4000 kg 4-氯邻硝基苯甲醚加到1500 kg水和15 kg1甲酸中,混合物加热至沸,并加入4000 kg 铁屑,加入速度在于保持希望的反应速度。当加入2000 kg铁屑或反应趋于缓慢时,再加入15 kg甲酸,甲铁屑时间10-12小时。
还原完毕,加入800 L溶剂石脑油,比蒸汽蒸馏可以得到较好的分离。静置15分钟后,将石脑油层排出。氧化铁用石脑油萃取2-3次,每次用800 L,石脑油萃取物合并,静置,过滤。水蒸汽蒸馏除去石脑油,然后将4-氯邻氨基苯甲醚 – 水混合物真空蒸馏处理。考虑到4-氯邻氨基苯甲醚的不稳定性,每次仅蒸馏小批量。4-氯邻氨基苯甲醚粗品熔点790C.
每吨4-氯邻氨基苯甲醚消耗:4-氯邻硝基苯甲醚1280 kg,铁屑1250 kg,甲酸10 kg,石脑油1000 kg(未计算回收)。
4-氯邻氨基苯甲醚(纯品): 铁粉还原法制备的4-氯邻氨基苯甲醚含有4%杂质,将其用石脑油重结晶除去杂质。 80份4-氯邻氨基苯甲醚粗品和20份溶解石脑油加热得到溶液,然后于约20小时内缓缓冷却到200C。母液由结晶器底部流出,然后结晶用10份新石脑油洗涤,放出洗涤溶剂后,纯的4-氯邻氨基苯甲醚熔融送往蒸馏釜,先蒸馏除去石脑油,然后蒸出纯的产品。蒸馏釜加入量应不大于500 kg,因为4-氯邻氨基苯甲醚不稳定。石脑油母液在水蒸汽蒸馏回收石脑油后弃去残液。粗品1170 kg回收1吨纯品,石脑油净耗100 kg。4-氯邻氨基苯甲醚熔点810C.
PB 70061, 955. 4-氯邻氨基苯甲醚产品标准。未抄录。
PB 70189, 6732. 4-氯邻氨基苯甲醚分析方法。分析号:286. 未抄录。
PB 70422, 2009-2013. Fast Red Salt RC. By Keller. 1937年12月13日。 (稳定重氮盐生产工艺)未抄录。
PB 70427, 6558. PB 73828, 5286.和 PB 74026, 2967. 4-氯邻氨基苯甲醚生产工艺。未抄录。
PB 74386, 16-17. 4-氯邻氨基苯甲醚 – 重氮钾盐生产工艺。1940年。未抄录。
PB 74386, 18-19. 4-氯邻氨基苯甲醚 – 重氮钠盐生产工艺。1940年。未抄录。
PB 82232, 699. Azorotsalz RC.(稳定重氮盐生产工艺) 2美元。美国人介绍如下。
From 4-chloro-2-anisidine and methylamino acetic acid.
不同稳定重氮盐,有不同用途,现补充如下:[68025-25-5]= C7H6ClN2O.3ClHZn
= 341.36.
[85252-22-8]= 2 C7H6ClN2O.1/2 Cl4Zn = 546.40.
上海市有机化学工业公司。《染料生产工艺汇编》1976年 。 p. 204-205. 红色基RC(2-甲氧基-5-氯苯胺盐酸盐)
2. 还原:(1. 甲氧基化: 请见CAS号[89-21-4], 已上网。)
在还原釜中加入17.5%硫化钠5800升,加热至1060C,搅拌,在1.5小时内缓缓加入甲氧基化物,并保持反应物沸腾回流,加完后,继续闷锅回流2.5小时,然后加水1000升,降温至950C,再静置30-45分钟,将下层物料放至铁盘中,冷却,结块,得红色基RC粗料。收率90%。
3. 成盐,精制:
在溶解桶中放入清水1800升,加入粗色基半批,400升30%盐酸,活性炭5公斤和陶土5公斤,用直接蒸汽加热至950C,保温40分钟。调整体积至3500升,加热至950C,保温1.5小时,然后静置2小时,将澄清液吸至盐析桶中,在搅拌下加入溶液体积20%的食盐盐析,自然冷却至470C,过滤抽干。收率97%。 总收率83%。(抄注:含甲氧基化。)
(本资料为内部出版物!)
徐克勋 主编。《有机化工原料及中间体便览》 辽宁省石油化工技术情报总站出版 1987年。P. 690-591.抄录如下。
2,5-二氯硝基苯,甲氧基化:略。
向还原釜中加入17.5%硫化钠,于1060C在1.5小时内加入甲氧基化物,加完料继续闷锅回流2.5小时,然后加水,降温至950C,再静置30-45分钟,将物料放至铁盘中,冷却,结块,得粗品;最后加盐酸精制而得成品,总收率83%。
徐克勋先生已开我们,但他已考虑到内部资料,不能照抄,所以只是摘录!
章思规 主编。《精细有机化学品技术手册》 科学出版社出版。1991年。 P. 197-198. 红色基RC 不再抄录。
资料全部抄自染料生产工艺汇编。未注明资料来源。 但它是正式出版物!如果说,英国人,美国人不说明资料来源,它至少是把德文译成英文了,
这里抄录德文的英文译文以及英文译文的日文译文和中文译文,其目的是希望读者学点外文,尽量要能看懂外文,否则人家译多少,你只能看多少,译错了,你也没有办法。不同译文可以各取所需,我是全抄(只是手头无德文原件),目的是请读者看看人家是这么编写生产工艺的(当然,这已是过去的历史资料)。
陈忠源 2017年6月29日 于 无锡 明辉国际。













