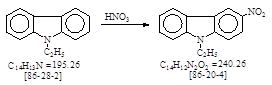
CAS号 [86-20-4] 生产工艺 3-硝基-N-乙基咔唑
CAS名: 9H-Carbazole, 9-ethyl-3-nitro- 历史参考文献: Beil. 20. E1, 168; E2, 288.
用途: 直接蓝108。颜料紫23。有机合成等。反应类别: 硝化。
生产工艺参考文献: 本人按手头资料整理如下。
BIOS 986, 72-73.(=胶卷PB 65657)Nitro-ethylimide base (Nitro-ethylcarbazole). 英国人译自德文(未提资料来源) 抄录如下。
反应式: 本人有加注。相对应德文原件暂未找到。
Plant: 设备:
1 Reaction vessel 1 cubic meter, cast-iron with jacket,
lined with silica tiles, anchor agitator (36 r.p.m.) and lid coated with V2A
Steel。 1 Pressure nutsch 1.5 sq. meter brick lined
with receiver。 1
Stone nutsch with spray. 2.5 sq. meter.
Method: 操作步骤:
200 kg. ethylimide base (N-ethylcarbazole) is added at
ordinary temperature to 180 kg. chlorobenzene (Note 1) and dissolved by stirring for 1 hour. Temperature 20-250C. 305 kg. 35.5%
nitric acid is added to the solution in 4-6 hours. Temperature 27-280C. (Note
2)(Cooling by water and / or brine). Subsequently the batch is stirred overnight
at 25-300C. A sample should
have m.p. = 129-1300C. (Test
a). Cooled to 100C. and
subsequently stirred at 100C. for 8-10 hours. Filtered off on the pressure nutsch and washed
3 times with about 10 kg. chlorobenzene.
The precipitate is then transferred to the stone nutsch and there washed
2-3 times with about 250 kg. of a 1% “Leonil”
SB solution (Test b) (Note 3). The paste is siterwards washed with 50 kg.
water. Dried in an air oven at 50-600C.
Yield of 3-nitro-ethylcarbazole is 78-80% theory. (抄注:Leonil 是萘磺酸甲醛缩合物类分散剂。)
The chlorobenzene is recovered from the original
filtrate and washings by steam distillation.
The residue contains explosive polynitrocarbazoles.
Tests: 生产控制点:
(a) Sample filtered off by suction, briefly
washed with chlorobenzene. Then twice with 2% “Leonil” water and finally neutral with water. Dried on porous pot. Melting point 129-1300C.
(b) For test of the completeness of the washing out of
the chlorobenzene with “Leonil” water, 100 g. of the filter cake is distilled with steam. No more than 3 cc. chlorobenzene should
appear in the distillate.
Properties: 原料与纯品物性:
Ethylimide base (N-ethylcarbazole from Mainkur) setting
point 64.5-650C., clear
solution in chlorobenzene.
The nitro-ethylimide base is a beautiful yellow. Melting point 128-1290C. Must
be completely neutral to Congo.
Notes on process: 操作注意事项:
1. Other solvents, such as nitrobenzene,
acetic acid, chloroparaffins, etc., were also tried but found less satisfactory. One
source gives the amount of chlorobenzne as 400 kg., but as the nitro compound
is rather soluble in chlorobenzene, the amount is reduced as far as possible. Formerly a small amount of sodium nitrite
was added to the chlorobenzene to start the reaction; later this was found to be unnecessary and is no longer used.
2. Another version states nitration is effected by 110%
of the theoretical amount of 30% nitric acid at 20-250C. Addition
takes 6-8 hr. The product is filtered straight away on the pressure filter, and
washed with a very small amount of chlorobenzene.
3. At present the adhering solvent is removed by
washing with cold water containing a dispersing agent (Leonil A) but previously the paste was transferred to a still and
the chlorobenzene, about 50 kg., steam distilled.
细田豊《理论制造染料化学》1957年。P. 792. 3-ニトロ-N-エチルカ- ハ”ソ”ル. 译自PB 65657. 抄录如下。
N-エチルカ- ハ”ソ”ル 200 kgをクロルヘ”ンセ”ン180 kgとませ”て溶し, 35.5%硝酸305 kgを20-280て4-6 hに加え,25-300て”1夜搅拌,100て”8-10 h搅拌後滤過し5), クロルヘ”ンセ”ン10 kg x 3回洗い, ヘ0- ストをスト- ンフイルタ- 移し, レオニ -ルBS 1% 溶液250 kgて2-3回洗い,水50 kgて”洗い, 50-600て”亁燥する。 收率78-80%。Mp 128-1290.
5) 滤液を水蒸汽蒸馏てクロルヘ”ンセ”ン回收,残渣は爆发性の多硝化カ- ハ”ソ”ル。
PB 73377, 2239-2240. Nitro-ethylimide Base
(3-Nitro-N-ethylcarbazole) 未抄录。
PB 73485, 2007-2008. Nitroethylimide Base. By Marschall 1936年。未抄录。
PB 73719, 2024-2031. Manufacture of nitroethylimde Base. 1938年1月3日。未抄录。
本胶卷中科编号Mo 4622. 沈阳院也有收藏。其中含1933年,1934年和1936年的德文生产工艺。
国内研究动态:
赵德丰 周丹红 杨锦宗(大工)。咔唑化学的进展及其在颜料工业中的应用。[J] 染料工业,
1987, 4. 32-38(65). 摘录如下。
N-乙基咔唑的硝化; 国外工艺。如用35.5%的硝酸,过量70%,于氯苯介质中进行硝化,需在25-300C反应12小时,然后保温8-10小时,加料时间需4-6小时。反应周期达24-28小时。(抄注:未说明引用文献。)。经实验,最后确定用微过量浓硝酸,在有机溶剂中,于低温下进行硝化。反应1.5小时即可完成,收率接近100%。
周丹红 杨锦宗 (大工)。 N-烷基咔唑的硝化及还原反应的研究。[J] 大连工学院学报(增刊)1988,65-72. 摘录如下。
将5.4 g N-乙基咔唑和50 ml 1,2-二氯乙烷置于125 ml的三口烧瓶中,搅拌,将温度降至100C, 在1 h 内滴加3 g 浓硝酸(d 1.38),然后在此温度下继续搅拌1 h, 当原料全部转化后,将反应混合物继续水蒸汽蒸馏,除去全部1,2-二氯乙烷。然后冷却,过滤。滤饼干燥后得到6.1
g粗产品,收率为92.6%,含量90.0%。将此产物用乙醇重结晶,熔点1230C。
大连工学院在国外发表的论文。[J] Dyes & pigment, 1995, 27(4) 287-296. 本人未收录。
王洪钟 刘亚华 (中标标准技术研究所)。3-硝基-9-乙基咔唑的合成研究。 [J] 染料工业, 1996, 5, 29-30. 摘录如下。
在装有搅拌器,温度计,滴液漏斗的三口烧瓶中,加入195.0 g 的N-乙基咔唑,487.5 g 1,1,1-三氯乙烷,搅拌溶解并冷却至00C,然后往滴液漏斗中加入30.0 g 浓硫酸及125.0 g 浓硝酸,进行滴加。滴加温度保持在0-50C,滴加时间为1小时。滴加完毕继续搅拌0.5小时。用稀氢氧化钠溶液中和至pH = 7.0, 然后过滤。滤饼用1,1,1-三氯乙烷洗涤,再分别用2%的拉开粉溶液及温水洗涤,抽干,然后干燥,得3-硝基-9-乙基咔唑216.3 g , 收率为90.1%,液相色谱仪分析其纯度为94.8%。
结果与讨论: 略。 参考文献: 5篇。
高文涛 赵德丰 杨锦宗(大工)。 三芳二噁嗪型荧光颜料 – 绿光紫的合成研究。[J] 染料工业,
1998, 4. 5-8. 摘录如下。
合成工艺与大连化工学院学报1988相同。
谢秋生 (鞍山热能研究院)。永固紫RL及其中间体的合成。 [J] 染料与染色, 2003, 4, 198-200. 摘录如下。
1.2.2 3-硝基-N-乙基咔唑的合成: 在500 ml三口烧瓶中加入300 ml 二氯乙烷,搅拌下加入40 g N-乙基咔唑,继续搅拌使之溶解。在100C下加入65% 浓硝酸30 ml,反应2.5小时即达终点。反应达终点后,将反应液水洗至中性,干燥得硝基咔唑43.6 g, 收率101.6%。 参考文献: 3篇。
随想: 以上资料如果没有多大参考价值,就作为学生学习参考资料吧!
陈忠源 2017年9月21日 于 无锡 明辉国际。













