CAS号 [121-73-3] 生产工艺 3-硝基氯苯
CAS名: Benzene, 1-chloro-3-nitro-历史参考文献: Beil. 5, 243; E1, 129; E2, 182; E3, 611; E4,
722。用途: 染料及医药中间体。反应类别: 氯化。
生产工艺参考文献: 按本人手头资料整理如下。
BIOS 986, 101-103. (=胶卷PB 77764) m-Chloronitrobenzene. (I.G. Griesheim). 英国人译自德文(未说明资料)。 抄录如下。
反应式: 本人有加注。本生产工艺分粗品,精馏和结晶三部分,德文原件暂未找到。
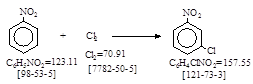
m-Chloronitrobenzene crude: Produced by direct chlorination of nitrobenzene.
3500 kg. dry nitrobenzene (or first runnings from the
fractionation of m-chloronitrobenzene) is chlorinated with dry chlorine at
35-40℃. in presence of 15 kg.
anhydrous sublimed ferric chloride until density of crude product reaches 1.35
at 15℃ The crude product is washed neutral with
water in a wooden vat, the washed product is then distilled 1 hr. with dilute
caustic soda and washed free from alkali.
Materials consumptions/tone of m-chloronitrobenzene
crude: Nitrobenzene 0.87 tone.
Chlorine 0.37 t.
Services consumption/tone of m-chloronitrobenzene
crude: Elecricity 274 K.W.H. Steam 1 t. H.P.
6 t. L.P.
Water 186 M3. Air
204 M3.
Plant for 24 tones/month of various chlorinated
products: 1. 3 x 4 M3 C.I. chlorinators, agitated, with
water jackets. 2. 1 x 15 M3 wooden vat.
m-Chloronitrobenzene pure (Ex chlorination of
nitrobenzene):
The crude chlorination product contains nitrobenzene,
m-chloronitrobenzene, a little o-chloronitrobenzene and some polychlorinated
nitrobenzenes, and is purified by fractionation and crystallization (“Sweating”).
(A)
Fractionation:
Fraction No.
Composition of fraction Disposal
of fraction
1. 1st runnings containing nitrobenzene Rechlorinated
2. Intermediate
fraction containing nitrobenzene and m-chloronitrobenzene Rechlorinated
3. Crude m-chloronitrobenzene Recrystallised or „Sweated“ for isolation
of pure m-chloronitrobenzene.
4. Tails Refractionated
5. Residue, o-chloronitrobenzene and
2,5-dichloronitrobenzene.
(B)
Crystallisation “Sweating“.
The crude m-chloronitrobenzene is charged to the “sweating”
plant, crystallized just below the crystallizing point and then cooled to 30℃. over a period of 24 hr. The temperature is then raised slowly and the
drainings collected until the latter reach C.Pt. 42℃.
The early drainings are combined with unaction 2 from the fractionation
for refractionation: the latter drainings are combined with the crude
m-chloronitrobenzene fraction from the fractionation for “re-sweating”. The pure product is then melted and
discharged from the “Sweating” apparatus.
Materials consumptions/tone m-chloronitrobenzene pure:
m-Chloronitrobenzene crude 1.98 tones, i.e. overall
yield from nitrobenzene = 45.5% theory (This yield low and probably does not
allow for return of the various fractions).
Plant: 1. Fractionation column 40 plates. 100 cm. diameter. 2.
Crystallising pan, vertical multitubular construction (see
trichlorobenzene pure) 4 M3 internal capacity of tubes.
m-Chloronitrobenzene pure (Ex ortho, para,
chloronitrobenzene nitration):
The crude fraction from para chloronitrobenzene
purification containing 72% m-chloronitrobenzene and 28% p-chloronitrobenzene
is treated with caustic soda to convert p-chloronitrobenzene to p-niyrophenol,
the m-chloronitrobenzene remaining unchanged.
The meta body is purified by fractionation and crystallization (“Sweating”).
2500 kg. of crude m- and p-chloronitrobenzene. 1800 l. water are heated to 140℃. (11 ats.) and 800 l. 400Be’ NaOH added in
ten lots. The pressure finally rises to
22-23 ats. (Due to presence of air used as oxidising medium) at 160℃.
The mixture is cooled to 80℃. and unchanged m-chloronitrobenzene drawn and
purified as previously described.
细田豊 《理论制造染料化学》 1957年。 P. 458. m-クロルニトロベンゼン. 译自PB
77764. 抄录如下。
ニトロベンゼン3500 kg + FeCl3 15 kgに35-400でCl2を通じ比重1.35 / 150とし,水洗後真空蒸馏した主馏分をスウュ-チングにかけ300から次第に上温して凝固点が420になるまで行う。
张澍声 《精细化工中间体工业生产技术》
1996年。 P. 105. 间氯硝基苯。 译自BIOS 986,
101.抄录如下。
在35-40℃将3500 kg干的硝基苯(或间氯硝基苯分馏的初馏份)用干燥的氯气氯化,并有15 kg升华的无水三氯化铁存在,直至粗品比重达1.35 (15℃)。粗产品在木槽中用水洗至中性,将洗涤的产品减压蒸馏,最后与稀NaOH溶液搅拌1小时,并洗至不含碱。
精制 – 粗氯化产品含有硝基苯,间氯硝基苯,少量邻氯硝基苯和一些多氯化的硝基苯,并用精馏和结晶进行精制。
(一)精馏: 第一馏份含硝基苯,用于氯化:第二馏份为中间馏份,含有硝基苯和间氯硝基苯,进行再精馏,第三馏份为间氯硝基苯,进行重结晶或发汗,使纯的间氯硝基苯不溶化:第四馏份为尾馏份,进行再精馏;第五为残留物,主要为邻氯硝基苯和2,5-二氯硝基苯。
(二)结晶(发汗): 间氯硝基苯粗品加到发汗盘中,在略低于结晶点进行结晶,然后在24小时内冷却到30℃。然后温度缓缓升高,并收集排出液体,直至排出液体温度42℃。 早期的排出液与用于再精馏的第二馏份合并,后期的排出液体与用于再发汗的间氯硝基苯粗品合并。
纯的产品熔融并从发汗设备中排放出来。每生产1吨纯的间氯硝基苯需使用1.98吨间氯硝基苯粗品,即以硝基苯计的总收率为45.4%。这一收率如此之低,似乎可能未计算各个馏份的回收部分。
在制备对位和邻位混合硝基氯苯的过程中,在精馏过程中,主馏份对硝基苯后有一中间馏份,该中间馏份含有72%间氯硝基苯和28%对氯硝基苯,将该中间馏份用NaOH处理,将对氯硝基苯转化为对硝基苯酚,而间氯硝基苯保持不变。2500 kg间和对氯硝基苯粗品和18000
L水加热到140℃(压力11巴),分10批加入800 L 35% NaOH溶液,压力最终升至22-23巴,温度16℃。混合物冷却到80℃,并抽出未变化的间氯硝基苯,如前法精制。
随想:已没有必要再多说,努力工作为新时代服务!谢谢读者Cited.
陈忠源 2017年11月21日













