CAS号 [576-24-9] 生产工艺。 2,3-二氯苯酚
CAS名:Phenol, 2,3-dichloro- 历史参考文献:Beil. 6. E1, 102; E3, 699; E4, 883.
用途:医药,如利尿酸。LookChem网登录生产与经营单位49家。
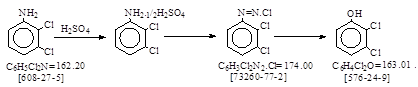
反应类别:氨基经重氮化水解转化成羟基。
BIOS 1153,
20-21.(=胶卷PB85687)。 2,3-Dichlorophenol. (Griesheim). 英国人译自德文(无资料来源)。
反应式:本人有加注,暂未找到德文原件。
Summary:
2,3-Dichloroaniline
is converted to the sulphate and is diazotized.
The diazo solution is run into hot sulphuric acid and the resulting 2,3-dichlorophenol
steam distilled off. The distillate is
treated with brine and the dichlorophenol separated as an oil.
The process
has been worked on the semi-technical scale at Grieheim on a few occasions and
is not developed.
Process:
Preparation
of 2,3-dichloroaniline sulphate: 2,3-Dichloroaniline 32 kg. (. 198 Mol. M.W. 162). Water
120 kg. Sulphuric acid 660Be’ 85 kg.
The sulphuric
acid is charged with agitation into the water, followed by the
2,3-dichloroaniline. The mixture is then
heated to 80-90℃. until the
base has dissolved.
The solution
is then quickly cooled so that dichloroaniline sulphate crystallizes out
in fine form. This is important as a small particle size is
essential for efficient diazotization.
Volume is about 170 l.
Diazotisation: Sodium Nitrite solution 50% 25 l.
The
suspension of the sulphate is then cooled to 0 to 5℃. and to it is added with efficient agitation
the sodium nitrite solution, at such a rate as to keep an excess of nitrite.
Hydrolysis: Sulphuric acid 660Be’ 45 kg.
Water 20 l.
A closed
agitated vessel is charged with the water and the sulphuric acid and the
mixture is heated to 120℃. The diazo solution is then
slowly in at such a rate that the temperature is 120℃. and the reaction is controlled (frothing).
During the
addition of the diazo-solution steam is evolved carrying with it the
2,3-dichlorophenol, and the distillate is collected in an agitated receiver.
When all the
diazo has been added the mixture is steam distilled until no more
2,3-dichlorophenol is evolved.
Separation:
To the
distillate is added with agitation, sufficient brine (210Be’) to give a separation
(25 to 50 l.). The mixture is heated to
600 to 70℃. and allowed
to stand when the dichlorophenol floats on the aqueous layer.
The aqueous
layer is run off from the bottpm, followed by the dichlorophenol.
Yield: About
171/2 kg. equivalent to 60% theory (M.W. 163).
Specification: The
2,3-dichlorophenol has a C.P. of 55℃.
张澍声 《精细化工中间体工业生产技术》1996年。P. 62.
2,3-二氯苯酚。 译自BIOS 1153, 20.
2,3-二氯苯胺转化为硫酸盐,并进行重氮化。重氮溶液流入热硫酸中,水解生成的2,3-二氯苯酚水蒸汽蒸馏出来。馏出物用盐水处理,二氯苯酚以油状物分出,该工艺当时在西德Grieheim厂以中试规模短时间生产,并未发展。
(一)2,3-二氯苯胺硫酸盐的制备:
在搅拌下将85 kg 98% 硫酸加入120 kg水中,随后加入32 kg 2,3-二氯苯胺,混合物加热至80-90℃,直至完全溶解。溶液迅速冷却,这样二氯苯胺硫酸盐以精细状态迅速结晶出来。这是很重要的,因为小的粒径对充分重氮化是非常主要的。体积约170升。
(二)重氮化:
硫酸盐的悬浮体冷却到0-5℃,在充分搅拌下向其中加入25 L 50% 亚硝酸钠溶液,加入速度要保持重氮液中有过量亚硝酸盐。
(三)水解:
在一密闭的搅拌釜中加入20 L水和45 kg 98% 硫酸,混合物加热到120℃。 向硫酸溶液中缓缓流入重氮液,流入速度要使温度维持在120℃,反应得到控制(泡沫)。在加入重氮溶液中,会发生水蒸汽,并带出2,3-二氯苯酚,馏出物收集在带搅拌的接受器中,当所有重氮物加完后,混合物水蒸汽蒸馏,直至不再有2,3-二氯苯酚馏出。
(四)分离:
在搅拌下向蒸馏物中加入足够的比重1.70的盐水,得到一个25-50 L的水层,混合物加热到60-70℃,静置,二氯苯酚浮在水层上,水层由底部放出,随后是二氯苯酚层。产品17.5 kg,相当于理论量的60%。2,3-二氯苯酚凝固点55℃。
国内出版物:
侯乐山 主编 《中国精细化工产品集 – 原料及中间体10396种》 2006年。P. 425.
2,3-二氯苯酚。
中国化工信息中心 全国精细化工原料及中间体行业协作组 出版。
生产方法:由1,2,3-三氯苯经磺化成盐,高压水解得3,4-二氯-2-羟基苯磺酸,再经硫酸水解去磺酸基而得。
抄注:无资料来源,也未提及上述历史生产工艺文献!
学习与思考:
从单元反应来说,氨基经重氮化,水解合成羟基是一种方法,例如本人介绍过的“共享”历史生产工艺 – 1. PB 25602, 764-766. “Paranol”
= 2-nitro-4-cresol. 就是由对甲苯胺经硝化,再将氨基经重氮化,水解法合成得到的,其CAS号[2042-14-0], LookChem网登录生产与经营单位有73家。未知读者是否见过上述德文生产工艺资料?
本人已准备上网“共享”历史生产工艺 – 2. PB 25625. 当然,只是作为参考材料,不可能全部抄录,只是希望引起读者的注意,因为有复印件,所以抄录美国人介绍的英文,中文只供参考(其中有一部分本人有德文抄录件,已上网!)。
我想多些参考资料总是有用的吧!(好在本人还有精力打字上网,如有错,请指正!)。再次谢谢读者!
陈忠源 2018年6月10星期日。













