CAS号 [6364-27-8] 生产工艺。 3-乙氧基-N-(4-甲基苯基) 苯胺
CAS名:Benzenamine, 3-ethoxy-N-(4-methylphenyl)- 历史参考文献:待检索。
用途:C.I.酸性紫15. LookChem网登录经营单位1家。 反应类别:羟基醚化。
BIOS 1153, 95-97.(=胶卷PB 85687)。 3-Ethoxy-4’-methyldiphenylamine.
(Ludwigshafen). 英国人译自德文(无资料来源)。
反应式:本人有加注。德文名:Ätoxy Base.
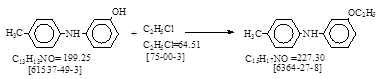
Outline:3-Hydroxy-4’-methyldiphenylamine is ethylated with ethyl chloride.
Plant: 设备:略。
Procedure: 操作步骤:
The autoclave (1) is charged with 500 kg. of 3-hydroxy-4’-methyldiphenylamine
ground to the size of hazel nuts, 400 kg. of recovered ethanol (benzene
denatured), and 104 kg. of fused caustic soda.
The autoclave is closed, heated to 60℃, and, with
the high pressure pump, 200 kg. of ethyl chloride forced in. The ethyl chloride is in a container under 3
atm. nitrogen pressure and is on a weighing machine. The temperature rises to 76℃. and the pressure to 4 atm., after 2 hours to 86℃. and 31/2 atm., and after a further 2 hours to 92℃. and 3 atm. It is then heated
to 100℃. and held for 24 hours when the pressure
drops still further to 11/2 – 2 atm.
At this temperature the ethanol is distilled off through a coil
condenser. The distillate (about 400 kg.
of 92% ethanol) can be used again. 500
l. of water are added to the autoclave and the contents blown to the separating
vessel (2). Some sodium chloride remains
in the autoclave and this is also washed into (2) with 100 l. of water. 70 kg. of caustic soda solution 500Be’ (Sp.
Gr. 1.526) are added and the mixture heated with live steam and stirring for 15
min., and then settled for 2 hours. The bottom
aqueous layer is run off to another vessel and the operation repeated with 50
kg. of caustic soda solution 500Be’ (Sp. Gr. 1.526), 500 kg. of water and 300
kg. of sodium chloride. The bottom
aqueous layer is run into the same vessel as before and from the whole, any
unchanged 3-hydroxy-4’-methyldiphenylamine is recovered.
The 3-ethoxy-4’-methyldiphenylamine is boiled in (2) with 2000 l. of
water and allowed to stand overnight. It
is tested for the presence of 3-hydroxy-4’-
methyldiphenylamine and, if satisfactory, the 3-ethoxy-4’-methyldiphenylamine
(now the bottom layer) is run to the drying pan (4) through a sight glass. The intermediate layer is retained for the
next batch, apply steam and vacuum for a long time, and when no more water
comes off, fill into drums.
The crude material is distilled at 6 mm. pressure and 200℃. Batch cycle time = 36 hours.
Yield: 450 kg. of crude 3-ethoxy-4’-methyldiphenylamine (≈ 420 kg.
distilled). 45 kg. of recovered
3-hydroxy-4’-methyldiphenylamine.
Observation: 观察要点:
1. The reaction temperature must not exceed 100℃. otherwise ethylation proceed on the nitrogen. 2.
The recovered ethanol used must be at least 85%. 3.
3-Hydroxy-4’-methldiphenylamine will not dissolve in caustic soda
solution of less than 5% strength.
Works Tests: 操作监测点:
1. For presence of 3-hydroxy-4’-methldiphenylamine: In a 2 l. flask, boil 100 g. of the
3-hydroxy-4’-methyldiphenylamine sample from (2) with 25 g. caustic soda
solution 400Be’ and 400 cc. of water.
Shake vigorously. Filter the upper
aqueous later through a folded filter paper and make the filtrate acid to pH 3
with hydrochloric acid. If the solution is
clear, no hydroxyl-compound is present.
If milky, cool in ice when if more than 0.5% hydroxyl-compound is
present the droplets will fall to the bottom.
If this happens, extract with ether, evaporate off the ether and
weigh. If more than 1% hydroxyl-compound
is present retreat with caustic soda solution.
2. Estimation of water in the
recovered 3-hydroxy-4’-methyldiphenylamine sodium salt: 100 g. Wet salt are dissolved in 300 ml.
water, acidified to pH 3 with hydrochloric acid, the oil separated off, dried
and weighed = % 3-hydroxy-4’-methyldiphenylamine. This number multiplied by 1.11 gives the
weight of sodium salt, and (100 – this number) is the % water. When this cake is re-used, care should be
taken that the water it contains does not dilute the ethanol to less than 85%.
Recovered Solvent: 溶剂回收:The recovered solvent contains about 6%
ether.
Final Product: 终产品: Can be tested as for 1 above.
Requirements and Services: 单耗及公用工程单耗:略。
张澍声 《精细化工中间体工业生产技术》 1996年。 P. 84-85. 3-乙氧基-4’-甲基二苯胺。 译自BIOS 1153, 95.
在1700 L衬铅带搅拌高压釜中加入500 kg 3-羟基-4’-甲基二苯胺(研细至榛子核大小),400 kg回收乙醇(苯处理)和104 kg 熔融氢氧化钠,关闭高压釜,加热至60℃,用高压泵压入200 kg氯乙烷。温度上升至76℃,压力达4巴;2小时后升至86℃,压力3.5巴,再经2小时升至92℃,压力3巴。然后加热至100℃,保持24小时,压力在降至1.5 – 2巴。
在此温度经冷却蛇管蒸馏出乙醇,蒸馏约400 kg 92% 乙醇,可以重新用,回收的溶剂含有约6% 醚。向高压釜中加入500 L水,然后一起压入5000 L锥形底分离器中,在高压釜中还留有一些氯化钠,用100 L水洗涤合并到分离器中。加入70 kg 50% 氢氧化钠溶液(比重1.526),混合物用直接蒸汽加热并搅拌15分钟,然后静置2小时。底部水层流到另一容器中,再加入50 kg 50% 氢氧化钠溶液(比重1.526),500 kg水和300 kg 氯化钠。底部水层再放入同一容器中。总的说来,所有未转化3-羟基-4’-甲基二苯胺已均回收。
3-乙氧基-4’-甲基二苯胺在分离器中与2000 L水煮沸,放置过夜,检验有无3-羟基-4’-甲基二苯胺存在,如已满意,3-乙氧基-4’-甲基二苯胺(现在为底层)流到干燥盘中。中间层留用于下次反应。在6 mm压力和200℃将粗产品蒸馏。得到450 kg粗品3-乙氧基-4’-甲基二苯胺和420 kg产品。回收45 kg 3-羟基-4’-甲基二苯胺。
反应温度不可超过100℃,否则将再单原子上发生乙基化反应。所用回收乙醇应至少是85%。每100 kg 产品消耗107.5 kg 3-羟基-4’-甲基二苯胺。
抄注:对照英文译文,有中文译文已不错了,未具体翻译的是原料与产品的分析方法,因为现有的方法比以前好多了!至于德文原件,未抄录,不能说明英文译文是否完全。本人上网的目的是希望读者能理解,是否有价值读者看吧!
PB 32533. Aromatic and
heterocyclic compounds. 共780页。 沈阳院有进口缩微胶卷。 中科院图书馆,编号:M01036.
PB 32533, II E. Ätoxybase =
3-Ethoxy-4’-methyldiphenylamine. 德文原件,未抄录。
国内出版物:
何岩彬 主编 《染料品种大全》 沈阳出版社 出版。 2018年。 P. 1851-2038. 染料中间体及可合成的染料。
p. 2003. N-对甲苯基间氨基苯乙醚。 上述资料可作为补充资料。
老有所为,为在能体现当前科技进步中的历史文献的《考古》!
陈忠源 2019年2月26日。













