CAS号 [86-79-3] 生产工艺。 2-羟基咔唑
CAS名:9H-carbazol-2-ol 历史参考文献:Beil. 待检索。
用途:冰染偶合组分,有机合成等。LookChem网登录生产与经营单位40家。 反应类别:见反应式(脱磺和环合)。
FIAT 1313, I, 362-364.(=胶卷PB 85172)。 2-Hydroxy carbazol. (I.G.
Leverkusen) 美国人译自德文,无资料来源。
反应式:本人有加注,2-羟基咔唑-3,6,8-三磺酸,暂未找到其CAS号。(资料中,Oxy-是德文 = 羟基)。暂未找到德文原件!
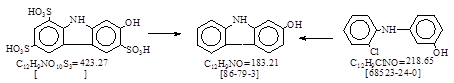
“2-Oxycarbazol No - 5633”。 3-Sulfonic acid groups are split from
2-Oxycarbazol-3,6,8-trisulfonic acid by heating with dilute sulfuric acid.
Apparatus: 设备:
1 – 5400 l. cast steel autoclave for 12 atmospheres pressure with tile(“Maxialstones”)of 65 mm thickness cemented with Höchst SW 10
cement and pointed with“Asppplit”cement.
The iron agitator is coated with“Asplit”.; 1 – Blow-case.; 1 – Nutsch.
Charge: 投料量:709 kg 100% = 100 kg Nitrit = 2-Oxycarbazol-3,6,8-trisulfonic acid
sodium salt, as paste.
120 kg sulfuric
acid 100% as 40% H2SO4 by volume.
Process: 操作步骤:
800 l. water and 180 l. 40% sulfuric acid (= 120 kg. H2SO4 100%) are
charged into the autoclave. Then 709 kg.
100% = 100 kg. nitrite, 2-Oxycarbazol-3,6,8-trisulfoniac acid sodium salt (as
Paste) is added. By blowing in steam of
13 atmospheres pressure, the pressure in the vessel rises in 3/4 to 1 hour to
10 atmospheres (temperature approximately 180℃). The charge is held at this
pressure for 15 hours. The air which
accumulates in the vessel is removed by careful deaeration.
Sampling: 取样分析:
A cooled sample of the Oxycarbazol is filtered; the acid filtrate is boiled
and then coupled under sodium carbonate alkalinity with meta-nitraniline
diazo. A liter of filtrate contains an
average of 1.6 g. nitrite equivalent of Oxycarbazol.
Finishing: 后处理:
Upon completion of the splitting-off reaction the pressure in the vessel
is released and the contents filtered hot on a nutsch. The 2-Oxycarbazol is washed acid free on the
nutsch with lukewarm water and a sample analyzed in the laboratory. It is then dried and ground.
Yield: 收率: 83 – 84% of theory.
细田豊《理论制造染料化学》。技報當 出版。1957年。 P. 650. 2-ヒドロキシカ-バゾル。译自PB 85172.
5.4 m3オ-トクレ-ブに40% 硫酸180 l + 水800 lを入れ前记オキシカ-バゾルトリスルホン酸100% 709 kgをペ-ストで加え,13气压の水蒸汽を吹入み1 hで气压(1800)に上げ15 h保温後压をぬいて滤過する。收率83 – 84%。
BIOS 1149, 114.(=胶卷PB 80376)。 2-Hydrocarbazole-3-carboxylic acid. (Leverkusen). 其中简要提到 [86-79-3] 的合成。不再抄录。
刘闪闪 程铸生(华东理工大学)。《2-羟基咔唑-1-甲酸的合成》。[J] 染料工业。1999, 4, 21-23. 其中含2-羟基咔唑的合成。
2.2 2-羟基咔唑的合成:
在1000 ml压热釜中投入11.6 g (0.05 mol) 2-氯-3’-羟基二苯胺和6 g (0.15 mol) 氢氧化钠,加入450 ml水,升温至260℃,压力为4.5 MPa,保温7小时。出料后用盐酸中和,产生大量灰色絮状沉淀,过滤,烘干得产品8.2 g,熔点220 – 254℃,产率89.5%。产品用减压升华提纯得橙黄色结体,熔点278.1 – 278.3℃。
IR (Nujol) cm-1: 3410, 3100, 1308, 1156, 810, 722。 UV (95% EtOH): λmax 235. 4nm。
1HNMR δ(CD3COCD3) (ppm): 7.94 (d1H) C – 5, 7.89 (d1H) C – 4, 7.41
(d1H) C – 8, 7.26 (t1H) C 7, 7.09 (t1H) C – 6, 6.94 (s1H)C – 1’, 6.76 (d1H) C –
3, 3.38 (2H) N – H O –H. 元素分析:略。 【参考文献】共7篇。未提及本人抄录的上述文献!
陈忠源 2020年5月5日星期二。 2020年5月11日星期一。













