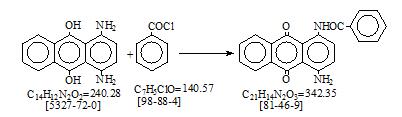
CAS号 [81-46-9] 生产工艺。 1-氨基-4-苯甲酰氨基蒽醌
CAS名:Benzamide, N-(4-amino-9,10-dihydro-9,10dioxo-1-anthracenyl)-
用途:还原染料中间体。LookChem网登录生产与经营单位8家。
BIOS 1484, 4.(=胶卷PB 86139)。 1-Amino-4-benzoylaminoanthraquinone(Leverkusen)。英国人译自德文。
Plant: (1) 1000 l. enamelled C. I. pan with jacket. (2) 4 sq. m. pressure filter. (3) 3 cbm. Venuleth.
Method:
100 kg. of leuco 1,4-diaminoanthraquinone is added to 700 l. of nitrobenzene at 100℃. 1 l. of piperidine is added as catalyst and the charge heated to 150℃. After cooling to 15℃., 60 kg. of soda ash and 2 l. of water are added, followed by 100 l. of benzoyl chloride. The temperature must not be higher than 45℃. The batch is cooled to 25℃., filtered on the pressure filter and washed twice with 250 l. of nitrobenzene. The cake is stirred with 250 l. of hydrochloric acid and 800 l. of water, filtered, washed four times with 500 l. warm water at 50℃. and dried in the Venuleth.
Yield = 90% of theory.
FIAT 1313, II, 25-6.(=胶卷PB 85172)。 1-Amino-4-benzoyl amino anthraquinone。 美国人译自德文。
This product is manufactured at Leverkusen by benzoylation of 1,4-diamino anthraquione, with benzoyl chloride in nitrobenzene. A brief description of the operating method is given below.
1000 kg. of leuco-1,4-diamino anthraquinone, 700 liters of nitrobenzene, 1 liter of piperidine.
The above ingredients are charged into a 1000 liter enamelled, jacketed, agitated kettle, equipped with steam and water for heating and cooling. The mixture is heated to 1500 and held under agitation until a sample examined under the microscope shows that oxidation is complete. When satisfactory the charge is cooled down to 150 and to the mixture are then added. 60 kg. of sodium carbonate, 2 liters of water.
The temperature of the mixture is now raised to 50 – 55℃., and the following previously prepared mixture of 70 kg. of benzoyl chloride, 140 kg. of nitrobrnzene is run in slowly during 2 – 3 hours, under agitation, keeping the temperature between 50 - 550 after which temperature is slowly raised to 600 and held at this point for 1 hour longer when the charge is tested for completion of the reaction, which is done by comparison of a sample dissolved in 50 : 50 mixture of pyridine and alcohol with that of satisfactory material similarly treated. This is also checked by microscopic examination.
When the reaction is satisfactory the charge is cooled to 250 and filtered off in an proof brick lined agitated pressure nutsch. The nutsch layer is first washed with two 250 liter washes of nitrobenzene and then slurried in the nutsch with 250 liters of hydrochloric acid(31%), 800 liters of water.
This slurry is then filtered and the nutsch layer repeatedly rinsed with four 500 liter water washes at 500, after which it is slurried with water and transferred to a 3 cubic meter Venuleth where it is dried.
Yield= 139 kg. of 1-amino-4benzoylamino anthraquinone. (90%) = 125 kg. of 1-amino-4-benzoyl anthraquinone (100% equiv.) = 87% of theory.
张澍声《精细化工中间体工业生产技术》。《染料工业》编辑部 出版。1996年。P. 233. 1-氨基-4-苯甲酰氨基蒽醌,译自FIAT.
在1000 L带搅拌的陶瓷衬里釜中加入100 kg 1,4-二氨基蒽醌隐色体,700 L硝基苯和1 L哌啶,混合物加热到150℃,在此温度下进行搅拌,直至取样在显微镜下检验氧化已经完全。混合物冷却到15℃,然后加入60 kg 碳酸钠和2 L水,升温至50 – 55℃。另将70 kg 苯甲酰氯和140 kg硝基苯预先准备成溶液,于2 – 3小时内在搅拌下缓缓加入,保持温度在50 – 55℃,然后温度缓缓升至60℃,在60℃保持1小时以上。取样溶解于50 :50吡啶和乙醇混合物中,在显微镜下检验,与标准品相同。
当反应已经满意后,冷却到25℃,过滤,滤饼先用250 L硝基苯洗涤,然后在抽滤器上用250 L 31% 盐酸和800 L水的溶液打浆,浆液进行过滤,并4次用500 L水洗涤,洗涤后,将浆料送入3立方米耙式干燥器中干燥。
得139 kg 90% 1-氨基-4-苯甲酰氨基蒽醌,相当于125 kg 纯品,收率为理论量的87%。
唐培堃(天津大学化工系)。《1-氨基-4-苯甲酰氨基蒽醌制备方法的探讨》。[J] 染料情报(天津),1983, 1, 18-20(41).摘录如下:
西德专利2,937,876(81, 4, 2)=(日开特 昭56-51439) 指出,用1,4-二氨基蒽醌隐色体为原料制备1-氨基-4-苯甲酰氨基蒽醌的方法存在很多缺点。并提出了用1,4-二氨基蒽醌为原料,在少量脂肪酸族叔胺醇或季胺化合物存在下进行单苯甲酰化的方法。
DE 2,937,876的实例1:在250 ml硝基苯中加入30 g 1,4-二氨基蒽醌(纯度91%),14 g 碳酸钠和0.2 g 三甲基-乙醇氢氧化铵(60% 水溶液),充分混合后,补加0.9 ml水,将混合物加热至35 – 40℃,用3小时滴加16 ml 苯甲酰氯,然后在100℃保温2 – 3 小时,再滴加不多于6 ml的苯甲酰氯。当蒲板色层分析分析指出只有痕迹量的原始反应物(<0.5%),并生成微量双苯甲酰化产物(<2%)时,即可认为反应已达终点。加入1 ml水,用减压蒸馏法蒸出硝基苯,得到65 g产品。产品中含有58% 1-氨基-4-苯甲酰氨基蒽醌。0.4% 1,4-二氨基蒽醌和1.5% 1,4-双苯甲酰氨基蒽醌。1-氨基-4-苯甲酰氨基蒽醌为理论量的94.%。
赵维绳 陈 彬 汪维凤 编著《还原染料》。化学工业出版社 出版。1993年。P. 97. 1-氨基-4-苯甲酰氨基蒽醌。
为了提高收率,可采用隐色体酰化法,即先将1,4-二氨基蒽醌还原为隐色体,再于硝基苯和少量哌啶溶液中,加热到150℃,使隐色体溶解,冷至15℃,加入碳酸钠缚酸剂,用苯甲酰氯酰化,过滤,用盐酸洗去为反应的二氨基蒽醌,可得1-氨基-4-苯甲酰氨基蒽醌,收率为90%。
何岩彬 主编《染料品种大全》。沈阳出版社 出版。 2018年。 P. 2030. 中文名称:1-氨基-4-苯甲酰氨基蒽醌。
【可合成的染料】C.I. 还原橙17;C.I. 还原红44;C.I. 还原紫16;C.I. 还原棕3:C.I.可还原棕3;C.I. 还原棕25;C.I. 还原棕84;C.I. 还原黑27:C.I. 还原黑29: C.I. 还原黑30;C.I. 颜料棕28。
陈忠源 2020年。/ 2021年6月9日星期三。













