CAS号 [85-56-3] 生产工艺 邻(对氯苯甲酰基)苯甲酸
CAS名:
Benzoic acid, 2-(4-chlorobenzoyl)- 参考文献:Beil. 10, 750.
生产工艺文献:
BIOS 987, 8-9. (=胶卷 PB 75860) 4’-Chloro-2-benzoylbenzoic
acid. 英国人摘译自德文。抄录如下。
反应式: 本人有加注:
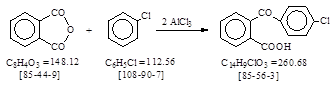
The fusion stage is batchwise, the isolation being
continuous.
3,000 kg. of chlorobenzene are charged into a 5,000 litre iron pan (flat agitator at 40 r.p.m.)
1,055 kg. of anhydrous
aluminium chloride are
added down a chute from the floor above in such a manner that the aluminium
chloride is not exposed to the atmosphere.
540 kg. of phthalic anhydride are now added over a period of 4 hours. The phthalic anhydride is fed
by means of a worm conveyor to a 6 inch
pipe down which the anhydride drops into the stirred mixture in the pan.
The temperature during addition is maintained at 75-800 by external cooling. After completion of the
addition the temperature is maintained at 75-800 for 1 hour.
The liberated hydrochloric acid is drawn away by fan
for absorption in a tower by water spray. The water passes from the tower to a
separator for chlorobenzene recovery.
The thick reaction mass is pumped via a valve at the
base of the pan into a porcelain tower (see sketch). Here the melt flow is met
by a stream of water which decomposes the aluminium
salt. The time taken to empty one pan is 6 hours. The resulting mixture is run into a porcelain separator.
This is so controlled that the chlorobenzene
layer is continuously passing to the next washing tank and the water layer
is passing to waste. In the washing vessel the chlorobenzene mixture is stirred
with excess water and passed on to the second separator . Here again the
controlled separation takes place and the chlorobenzene mixture passes on to be
treated with caustic liquor. In this vessel in which the mixture is kept just alkaline,
the chlorobenzoylbenzoic acid is
extracted from the chlorobenzene as the soluble sodium salt. The separated
chlorobenzene is run over caustic soda limps to remove traces of water and from
there to a continuous still for re-distillation and further use.
The sodium salt solution is pumped into the acidification vessel where it impinges
on to a stream of 10% sulphuric acid at
such a rate that an acidity to Congo Red is always maintained. The temperature of precipitation is about 250.
The acidified mixture drops to a rotating vacuum filter
on which it is filtered and washed. The filter cake falls from the filter drum
down a chute into a special dryer. This is a large tile lined vessel containing
a series of circular shelves with numerous slots. The drying powder is made to
drop from shelf to shelf by a series of revolving rakes, being dried by a
current of hot air. The dry finished product passes from the base of dryer to
metal drums.
Yield =
900 kg. = 95% theory. M. P. 148-1500C. Loss of solvent is about 10%
Sketch of 4’-Chloro-2-benzoylbenzoic
acid lay-out. 略!请见原书。未说明资料来源,为什么?
FIAT 1313,II, 37-9. (=胶卷 PB 85172) Chloro benzoyl benzoic acid
(CBB). 美国人摘译自德文。抄录如下。
The Friedel-Craft reactions involved were carried out
in two 6,000 liter cast iron kettles, operated alternately so that a continuous supply of finished condensation mass
could be furnished for isolation equipment which was designed to operate
continuously starting with the drowning of the condensation mass through to the
dry product, Equipment for ring closing the products was also installed in this
building.
The capacity of these sets was very large, being rated
at four batches per day of 900 kg. each,
or 3.6 metric tons per day, the rate being approximately the same for
either product. Actual capacity rates were realized in 1939 when 1124 metric tons
of CBB and 199 tons of PTB were
manufactured in the equipment. The charge for CBB was as follows:
3000 kg. of chloro
benzene. 1053 kg. of aluminum
chloride. 540 kg. of phthalic
anhydride.
The operation in brief was
as follows: The kettle
was charged with the dry chlorobenzene and all of the aluminum chloride was
then added. It was found necessary that good quality; i.e. fresh, coarsely
ground anhydrous aluminum chloride, be used since, otherwise, the use of old
aluminum chloride generally resulted in a thick condensation mass and if
unground material were charged troubles with plugging usually resulted.
Following the addition of the aluminum chloride to the
solvent the temperature of the mixture was adjusted between 75-800C and addition of
phthalic anhydride was begun. The phthalic anhydride was added by means of a
screw conveyor type of charging apparatus, a plug of phthalic anhydride always
being present in this apparatus. Approximately four hours were required to feed in the phthalic anhydride, the
temperature being held between 75-800C. by means of cooling water in the jacket of the kettle. When cgarging was
complete, the charge was held for one
hour longer at the same temperature and then sampled for completion of the reaction.
A small 5 cc.
sample was withdrawn and diluted with 10
cc. of chlorobenzene in a test tube after which 5 cc. of dimethylaniline was added. The test tube was then warmed
in a water bath to 900 and 5
cc. of pyridine added. A green color
formation (Fischers Green) indicated the presence of unreacted phthalic
anhydride, whereas a completed reaction mass gave only an olive brown color.
When the reaction was complete, the charge was pumped
from the bottom of the reaction kettle into the middle section of a porcelain
decomposition tower by means of a motor driven reciprocating piston pump. The
speed of this pump could be regulated so that a regulated stream of 10% sulfuric acid was added in
sufficient amount to decompose the reaction product continuously within the
tower with evolution of a constant stream of hydrogen chloride. The gas was
drawn off and absorbed above and into a porcelain tower equipped with water
jets, by means of a porcelain fan, while the drowned slurry ran off below
through a porcelain solvent separator which separated the solvent layer
containing the product continuously, the aqueous acid layer being run to the
ditch. The solvent layer was then washed with water continuously and again
separated in a second porcelain separator after which it flowed through a steel
alkaline extractor where the product was dissolved continuously from the
solvent layer as the sodium salt with 5%
caustic soda solution.
The extracted solvent layer was then again separated in
a steel separator following which it was dried by passage through a column
packed with dry caustic soda and finally run through a continuous solvent
recovery still and run to a dry solvent storage for reuse in the process. The
alkaline aqueous layer containing the product was then passed continuously
through an acid precipitator fed with a continuous stream of 10% sulfuric acid and the precipitated
slurry run to a rubber covered rotary filter upon which the product was
continuously filtered off, washed and discharged to the hopper of a porcelain
lined continuous (Teller) dryer from which it was discharged to storage
containers.
The 10% sulfuric
acid used in the process was derived from ring closure of the CBB to 2-chloro anthraquinone which was
carried out in an acid ring closing set installed in the same building and
arranged for collection of the filtrates from this operation.
Normal yield from 540
kg. of phthalic anhydride was 900 kg. of CBB or about 95% and the melting point of the product was given as 148-1500C.
It was stated that, innormal operation, the solvent loss was about 10%.
未说明资料来源,不知道为什么?
日文摘译文。 细田豊《理论制造染料化学》 1957年。P. 552-3. 其参考是BIOS和FIAT。请见原书。
中文摘译文。 张澍声《精细化工中间体工业生产技术》 1966年。P. 226. 译自BIOS和FIAT。请见原书。
PB 25623, 381-6. Fabrikationsvorschrift Chlorbenzsaeure. (用于生产2-氯蒽醌)未抄录。
PB 25627, 2473-4. Fabrikationsvorschrift chlorbenzsaeure (用于生产还原红10)1940年1月17日。抄录如下。
Fabrikationsvorschrift: 操作步骤:
In den Reaktionskessel werde 2800 kg. Chlorbenzol rein und 1250 kg. Aluminumchlorid K eingefuellt, auf 450 aufgewaermt und bei 45-600 in ca, 4
Stunden 500-520 kg. Phtalsaeureanhydrid 1 mal dest. (tel quel) eingefuellt
dann eine Stunde bei 650, 1
Stunde bei 750 gehalten. Weitere Aufarbeitung und Umloesung wie
bei T-saeure. Das Ausfaellen der
umgeloesten und filtrierten Chlorbenzsaeure geschieht bei 750,
dann wird auf ca. 300 heruntergekuehlt
und abgenutscht.
Trocknen der Chlorbenzsaeure im Venuleth mit 1.5-2 atue Dampf. Probe
vor dem Ablassen,wie bei T-saeure.
Ausbeute: 100 kg.
Phtalsaeureanhydrid 100% geben 165-170 kg.
Chlorbenzsaeure 100% = 93.7-96.6% der Th.
Untersuchung
der Chlorbenzsaeure: 氯苯甲酰基苯甲酸的分析测定:
a) Trockenbestimmung: ca. 10 g. (genau gewogen) Chlobenzsaeure werden 3 Stunden bei 1000 getrocknet. Der Trockengehalt soll nicht unter 99.8% betragen, andernfalls muss die Partie nachgetrocknet werden.
b) Rueckstandsbestimmung: 50 g. Ungeloester Chlorbenzsaeure werden in 500 ccm Wasser und 30 ccm Ammoniak 25% gekocht, durch
gewogenes Filter filtriert. Der ausgewaschene und getrocknete unloesliche
Rueckstand soll nicht ueber 0.1-0.15 g betragen.
c) Schmelzpinkt: Smp. 147-1500
Pruefung
der Ausgangsmaterialien: 原料分析:
Phtalsaeureanhydrid halbfertig K. Die Ware muss absolut
wasserfrei sein, was bei den laufenden Partien auch der Fall ist.
Phtalsaeureanhydrid ist – besonders in feiner Vertielung – hygroskopisch;
laengere Aufbewahrung (ueber 2-3 Monate) ist tunlichst zu vermeiden. Pruefung
aud Wasser nach Methode “Aufhaeuser“. Der Reingehalt wird vom Lieferabten durch
Titration festgestellt und betraegt beim Phtalsaeureanhydrid halbfertig K 90-93%. Rest: Cips (1%) und
Kondensationsprodukte aus Phtalsaeyreanhydrid und Naphtochinon bezw.
Verkohlungen.
Aluminumchlorid K. Die Ware soll aus
braunroten Kristallen bestehen, Feines Pulver, das stark raucht (Titanchlorid)
und stark nach Phosgen riecht, ist nicht geeignet. Gehalt an: Titanchlorid 0.5-1%, Eisenchlorid
hoechstten 4%, Phosgen O –Spuren. Der Hersteller bestimmt den Wert des
Aluminumchlorids nach der Waermpruefungsmethode und stellt ausserdem den in
Wasser unloeslichen Rueckstand fest. Ungenuegende Partien gelangen nicht zur
Ablieferung.
PB 70057, 8327-35. Fabrikationsvorschrift Chlorbenzsaeure 1945年9月3日。(这是德文工艺原件。)摘译如下。
本生产工艺规程,前部略。
B. 操作步骤:1. 缩合:
由落地氯苯贮槽,用水环泵经流量计,向位于二楼(Lu 东侧)的二台缩合锅分别打入3000公斤 无水氯苯,在半小时内将其加热(用夹套蒸汽)到40-450C, 然后通过三楼破碎机,经加料管加入1053公斤无铁三氯化铝,从40-450C 开始在4小时内通过三楼配有铁齿的Alexander磨,经加料管均匀地加入540 公斤苯二甲酸酐(*要注意,无论是加三氯化铝或是苯酐,都要通过料斗计量后加料),因为是放热反应,所以加苯酐时温度会升高到75-800C (注意冷却!),加完在75-800C 保持一小时(取样1.)。
从加三氯化铝开始到分解完毕,应始终打开三楼(东侧)陶瓷通风装置,使盐酸气通过陶瓷管进入有喷水的陶瓷吸收塔(它装有四个喷水器),出来的气体再通过同样的陶瓷吸收塔,随气体带出的氯苯用喷洗分离器分离。
2. 络合物分解:
将缩合物从锅底放出,用拉杆泵迅速打入陶瓷分解塔,物料应在6-6.5小时内打完,分离塔高6.5米,直径1米,中间有陶瓷盘,物料从一侧进,另一侧进10% 硫酸(一份物料对一份稀硫酸),盘底装有锥形陶瓷,使要分解的物料在塔内有足够的停留时间。85-900C 的物料经分解后流入装有拉希环的700升分离器,上层是氯苯甲酰基苯甲酸的氯苯液,下层是三氯化铝分解液,它放入立式平底受槽内,分出少量氯苯送Blankorol厂回收铝。(取样2.)
氯苯甲酰基苯甲酸料液进入有Hoe搅拌,450转/分的465升洗涤锅,用热水洗去铝盐,每批再用热水彻底洗净,放入陶瓷分离器,此时物料仍在上层,洗水在下层。
经洗涤分离的物料进入转速120-180转/分,有五层浆式搅拌的萃取器下部,与此同时从塔底进入1.5%的液碱,塔内温度为800C, 每批物料约需100% 氢氧化钠150公斤,由萃取器上部连续流出的物料进入700升提纯分离器,此时上层分出的是氯苯甲酰基苯甲酸钠盐溶液,下层是氯苯(取样3.)
氯苯流入6立方米贮槽,用离心泵打入管式蒸发器(二楼)进行蒸馏,馏出液通过冷凝器进入250升分离器(分出水),而后进入内装有固体碱的2台氯苯干燥塔,每台700升,干燥后送氯苯贮槽。
从提纯分离器出来的氯苯甲酰基苯甲酸钠盐溶液进入2台缓冲器,陶瓷搅拌,转速36转/分,容量9900升,再进铁分离器分出微量氯苯,经分离后用拉杆泵打到离析器(二楼),在25-300C,用10% 硫酸酸化析出氯苯甲酰基苯甲酸,酸化是控制到刚果红呈酸性(取样4.)
离析料用大型过滤器过滤,面积为1.5平方米,水洗到中性,(取样4.)滤饼用带式输送器送到Bruther干燥器干燥,
每批得量:907公斤。 收率: 95.5%。
本生产工艺规程,后部略。
国内工艺汇编:上海,p. 63-4. 天津,p.
58-9.(国内是参照BIOS和FIAT开发的。)
加注:抄录英国人摘译文,美国人摘译文以及德文工艺原件本人的翻译,是要说明原件的优点,另外各人理解不同,译文也会有所不同,问题是过去不知道,今天补上,供参考。请利用原件!国内有此类原件!
陈忠源 2016年8月19日 于 无锡 明辉国际。













