CAS号 [6401-98-5] 生产工艺 1-苯基-3-甲酰氨基-5-吡唑啉酮
CAS名: 1H-Pyrazole-3-carboxamide, 4,5-dihydro-5-oxo-1-phenyl- 历史参考文献: Beil. 24, 154.
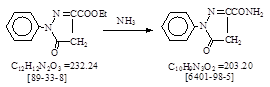
用途: 媒介红71。碱性染料和金属络合类酸性染料。反应类别: 酯交换。(-COOC2H5 à -CONH2)
生产工艺文献: 按本人手头资料整理如下:
BIOS 1153, 346-347.(=胶卷PB 85687) 1-Phenyl-5-pyrazole-3-carboxylamide. (Hoechst). 英国人译自德文。抄录如下。
反应式: 本人有加注,英国译者和美国译者均未说明资料来源。
Plant: 设备: 以下抄录不再分项。
Cast steel autoclave, 1000 l. capacity, heated by steam
coil. Tested to 10 atm. working pressure. Storage vessel and measure vessel for 25% ammonia. 2 Blow-eggs. Clarification press. 1 Precipitation vessel. 1 Wooden nutsche covered twill.
Tiled-lined vacuum
receiver. Absorption system for
recovered ammonia.
Materials for 0.8 kg. mol. Charge: 0.8公斤分子投料量:
185.6 kg. 1-Phenyl-3-carbethoxy-5-pyrazolone dry 100%.
M.W. 232. 138.0 kg. 100% ammonia = 552
kg. 25% aq. Ammonia.
130.0 kg. Crude hydrochloric acid. Recoveries: ca. 104 kg. 100% ammonia
as 415 kg. 25% aq. Ammonia.
Process: 操作步骤:
The autoclave is charged with the
1-phenyl-3-carbethoxy-5-pyrazolone followed b y the aqueous ammonia from the
storage vessel via the measure vessel. The autoclave is sealed and well
stirred, whereupon the temperature rises to 35-400. It is heated to
800 with agitation and held for 6 hours at 80-850. The
pressure is 4.5 atm. at the start falling at the end to 3 atm. The autoclave is
then vented through the absorption system to recover the excess ammonia and
heated during 8 hours to 95% to expel all the ammonia. The autoclave contents
must now smell only slightly of ammonia.
The brownish liquid is now blown into 1500 l. of cold
water and the volume made up to 2400-2500 l. The residue is removed by passing
the solution at 300 through a clarifying press, and is washed with a
little cold water. The filtrate is blown to the precipitation vessel and
stirred at 300 whilst 130 kg. of crude hydrochloric acid are run in
to precipitate the product. Sufficient acid is added to give a slight Congo
acidity after several hours stirring; further addition should give no further
precipitation. The product is filtered and washed acid-free on the nutsche with
cold water.
Yield of 1-phenyl-5-pyrazolone-3-carbxylamide = 156-158 kg. @ 100%. M.W. 203 as a
20-22% paste. = 96-97% theory.
Strength is determined by coupling with diazotised
p-nitroaniline. About 104 kg. 100% NH3 (as 25% aq. NH3)
are recovered.
Nitrogen analysis of the dried product gives 19.34% N
(theory = 20.69%) equivalent to 93.5-94% strength.
FIAT 1313,I, 238-239.(=胶卷 PB 85172) 1-Phenyl-5-pyrazolone-3-carboxylic acid amide.(Hoechst)美国人译自德文,抄录如下。
Apparatus: 1 – cast steel agitated bomb (10 atmospheres
pressure). 1 – adsorption tower for
ammonia.1 – 3,000 l. vat.
1 – filter press. 1 – 3,000 l. precipitation vat. 1 – nutsch.
Charge: 投料量: 185.6
kg 1-phenyl-5-pyrazolone-3-carboxylic acid-ethyl ester, mol.wt. 232.
352 kg aqueous ammonia
25%. 130 kg hydrochloric acid
technical.
Process: 操作步骤:
185.6 kg of 1-phenyl-5-pyrazolone-3-carboxylic
acid-ethyl ester, dry material 100%, mol. Wt. 232, are charged into a cast
steel agitated bomb (10 atmospheres working pressure). 352 kg of aqueous
ammonia 25% are then added from a storage vessel through a measuring vat. The
bomb is sealed and the material mixed thoroughly by stirring whereupon the
temperature rises to 35-400C. Under agitation the charge is heated
to 800C and held at 80-850C for 6 hours. In the beginning
of the reaction the pressure is about 4.5 atmospheres and at the end of the
reaction about 3 atmospheres. The gas is then permitted to escape slowly from
the bomb and to the adsorption tower to recover the excess ammonia. Then the
bomb is heated to 950C for about 8 hours in order to drive off the
remaining excess ammonia to the adsorption tower. At the end of this period the
contents of the bomb should still smell strongly of ammonia. The
brownish-colored liquor is then forced to a 3000 l. vat containing 1500 l. of
cold water and the combined liquor and water adjusted to a volume of 2400-2500
l. The solution is then filtered thru a clarifying press to free it from
residue. The residue in the press is washed with a little cold water. The
filtrate is run to a 3000 l. precipitation vat and under agitation at a
temperature of about 300C, the pyrazolone carboxylic acid amide is
precipitated by running in about 130 kg of hydrochloric acid. Sufficient
hydrochloric acid is added to obtain, after several hours thorough agitation
and mixing, a distinct position reaction to Congo red. The point of complete
precipitation is reached when additional hydrochloric acid added to the
filtrate of a test sample shows no further precipitate. The phenyl-pyrazolone-carboxylic
acid amide is then filtered on a nutsch and washed acid free with cold water.
Yield:
156-158 kg 1-phenyl-5-pyrazolone-3-carboxylic acid amide 100%, mol. Wt.
203, in form of a 20-22% paste.
Notes: The
yield, determined by coupling with diazotized para-nitraniline, is 84-85.1% on
the basis of the carbethoxy pyrazolone employed and equal to 96-97.1% of
theory. About 415 kg of ammonia 25% strength, equivalent to 105 kg NH3 100%, are recovered in the adsorption tower. On the basis of nitrogrn analysis
of a salt free washed and dried sample, the product is about 93.5-94% pure.
张澍声 编译。《精细化工中间体工业生产技术》 1996年。P. 245-246. 1-苯基-5-吡唑啉酮-3-羧酸胺。译自英文。抄录如下。
在铸钢带搅拌高压气罐(工作压力10巴)中装入185.6 kg 100%干品1-苯基-5-吡唑啉酮-3-羧酸乙酯,再加入352 kg 25%氨水。将气罐封闭,物料充分搅拌混合,同时温度上升至35-400C。在搅拌下将物料加热至800C,在80-850C保持6小时。在反应开始时压力约为4.5巴,在反应末期则为3巴。然后让气体从气罐中缓缓逸出,在吸收塔中回收过量的氨,然后将罐加热到950C经8小时,将其余的过量氨赶跑到吸收塔中。在吸收末期罐内所含物仍然嗅到强烈的氨味,将这种棕色液体压入含1500 L水的3000 L槽中,调整体积到2400-2500 L。溶液经澄清压滤机过滤,使不含残渣。压滤机中的残渣用少量冷水洗涤。滤液流入3000 L沉淀槽中,在约300C及搅拌下加入约130 kg工艺盐酸以沉淀吡唑啉酮羧酰胺,经数小时彻底搅拌混合,对刚果红为明显酸性,说明加入足够的盐酸。如果取样过滤,滤液再加盐酸,无沉淀产生,则沉淀已经完全。然后抽滤苯基吡唑啉酮所酰胺,用冷水洗至不含酸。
得到156-158 kg 100% 1-苯基-5-吡唑啉酮-3-羧酰胺,为20-22%膏状物。收率96-97.1%。 在吸收塔中回收415 kg 25%氨水,相当于105 kg 100% NH3。 通过氮分析,产品纯度约93.5-94%。
PB 25602, 772-774. 1-Phenyl-5-pyrazolone-3-carboxylic acid
amide. 德文生产工艺原件。(未抄录年份)未抄录。
PB 70150, 467-469. 1-Phenyl-5-pyrazolone-3-carboxylic acid
amide. 德文生产工艺原件。(未抄录年份)未抄录。
PB 70361, 6693-6694. Phenylpyrazolonecarboxylamide moist. 美国人介绍如下。1.5美元。未抄录。
(1-Phenyl-5-pyrazolonw-3-carboxylamide)By Waldmuller. 1934年5月9日。德文。
抄录说明:
抄录英国人和美国人的译文,说明同一德文原件有两种译法,而且都没有说明资料来源!另一方面说明酯交换法,在1934年已有生产工艺。
本产品的应用开发,可从CA中检索到,已有不少专利。
我在已上网的资料中,有的提到未找到德文原件,主要是因为特种文献没有详细得目录。有一些,美国人也没有介绍。当然,这些资料用处不大,仅供参考,到此为止。
陈忠源 2017年7月4日 于 无锡 明辉国际。













