CAS号 [92-36-4] 生产工艺 脱氢硫代对甲苯胺
CAS名: Benzenamine, 4-(6-methyl-2-benzothiazolyl)- 历史参考文献: Beil. 27, 376.
用途: 碱性黄1。直接红3, 11, 20, 47, 48。硫化黄4。颜料黄18。荧光增白剂41。反应类别: 硫氢化,缩合,闭环。
生产工艺参考文献: 按本人手头资料整理如下:
BIOS 1153, 349-355.(=胶卷PB 85687)Dehydrothio-p-toluidine [2-(p-Aminophenyl)-6-methylbenzthiazole] (Mainker) 抄录如下。
反应式: 英国人译自德文,译者未说明德文资料原件,本人按德文原件列出反应式,同时有加注。
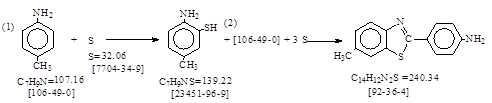
Summary:
p-Toluidine is fused with sulphur, and, after
completion of the reaction, the excess p-toluidine distilled off. The recovered
p-toluidine is used in subsequent manufacture.
The crude dehydrothio-p-toluidine is then distilled
and, when solidified, broken up for alcoholic extraction. The alcoholic treated
product is filtered on a pressure filter and finally dried. The alcohol used is
recovered by distillation and re-used in the process.
The hydrogen sulphide given off in the initial fusion
is passed to a recovery unit for conversion to sodium hydrogen sulphide.
Raw Materials: p-Toluidine
Technical quality Crystallising point above 42.50C. Sulphur Technical quality in form of
powder.
Process: (a) Condensation: Charge: 1240 kg. of p-toluidine. 445 kg. of sulphur. 2 to 3 kg.
of soda ash
The above mixture is charged into the fusion pot and is
heated to 1800C. in about 1 hour with agitation.
The temperature is then raised from 180 to 1900C.
in about 31/2 hours; from 190 to 2000C. in another
3 to 4 hours and from 200 to 2100C. in a further 3 to 4 hours. The H2S
is evolved at about 1800C. and continuous to 2200C. It
passes to the absorption unit. When 2200C. is reached the
temperature is slowly raised to 2250C. in another 3 to 4 hours.
Total time of melt is 18 to 20 hours.
(b) p-Toluidine Recovery: The
melt is then sucked over into a still and vacuum slowly applied. The
temperature of the batch, which is at 180-2000C. on reaching the
still, falls in about 3 hours to 1200C. and the vacuum reaches 5 to
10 mm. on the still body.
The p-toluidine comes over and when the temperature on
top of the entrainment head reaches 125-1300C. the distillation is
complete. The still body temperature rises to 1500C. The total time
of the distillation is 10 hours.
p-Toluidine recovered is 480 kg. This is employed in
subsequent batches. It is removed from the receiver by being broken up when
cold. And is transferred to drums. Recovery of p-toluidine = 38.7% of initial charge.
(c) Distillation of crude:
The melt is then sucked into another still and heated
to 1800C. under 4 to 5 mm. vacuum. The distillation is complete when
the temperature at the top of the small entrainment column is 240-2500C. (With bad vacuum this can be as high as 3000C.). Total time for distillation 10 to 12 hours.
There is a residue of 50 to 100 kg. Vacuum is broken
with nitrogen and the residue ladled cut of the still by hand into trays. This
goes to waste.
The distilled dehydrothio-p-toluidine is solidified in
the receiver and when the temperature is below 1000C. is broken up
after removal of the large man lid and transferred to open drums (M.P. 150 to
1540C.).
(d) Alcoholic extraction:
The product is then charged into the extractor which
has previously been charged with 500 l. of ethyl alcohol. This is refluxed and
stirred at 600C. for about 1 hour then cooled to room temperature.
The impurities in the product are thus dissolved in the alcohol leaving the
dehydrothio-p-toluidine in suspension.
(e) Filtration:
The batch is then blown to a pressure filter and
filtered. Air is used. The batch is given
a first wash of 50 l. of alcohol, followed by two or three washes until the
filtrate is clear. The product is then blown to force entrained liquid through.
(f) Drying:
The batch is then transferred to an agitated enclosed
dryer, and the alcohol distilled off with steam heating.
Yield =
470 to 480 kg. dehydrothio-p-toluidine = 57% theory. Recovery: Sodium hydrogen
sulphide 350 to 380 kg.
Specification of finished Product: The
C.P. is 180 to 1920C
Materials and Expenses Consumption per 100 kg.
dehydrothio-p-toluidine:
p-Toluidine 156
kg. Sulphur 92 kg. Soda ash 0.6 kg. Ethyl alcohol 11 kg.
Steam 1.5
tones. Electricity 68 kW-hr. Gas 132 M3. Water
40 M3. Wages 7 man
hours.
Plant and Operation: 设备与操作要点: 略。
FIAT 1313, I, 126-127.(=胶卷PB 85172)69. Dehydrothio-p-toluidine. (I.G. Mainkur). 美国人摘译自德文。抄录如下。
Dehydro Thio Para Toluidine was isolated from the
fusion mass by vacuum distillation and then purified by crystallization from
alcohol. An outline of the processes obtained directly from the chemist in
charge is: Dehydro Thio Para
Toluidine. All equipment was iron. Charge: 1200 kg para Toluidine. 430 kg sulfur. 1 – 2 kg sodium carbonate (to neutralize
acid in sulfur).
Heat rapidly (2-3 hours) to 1800C. Then raise
temperature 30 per hour until evolution of hydrogen sulfide ceases.
Heat to maximum temperature of 220-2220C. Time 18 – 20 hours.
Transfer to vacuum still to recover para toluidine.
Without applying external heat, gradually build up vacuum, meanwhile distilling
off para toluidine until still temperature falls to 1500C. Take off
para toluidine up to a vapor temperature 110-1150 at 2-3 mm.
Recovery:
470-480 kg para toluidine.
Transfer to a second vacuum still. Distill off 40-50 kg fore-shots up to
a vapor temperature of 2000C. Then distill the dehydro thio para
toluidine at 2-3 mm. Yield: 600-610 kg dehydro thio para
toluidine crude.
Add 600 kg of this crude to 600 liters alcohol and heat
to 50-600C. Cool to 200C and filter. Wash with 400-500 l.
cold alcohol. Dry the purified dehydro thio para toluidine in a graining
bowl. Yield: 470-480 kg. Freezing
point 190-1920C.
The recovered para toluidine was used again in
subsequent charges. The foreshots from
the distillation, the residue from the still, and the residue obtained in
alcohol recovery from filtrates were all discarded. Considerable study had
shown these materials to be of no value.
细田豊。《理论制造染料化学》 1957年。P.
514. テ”ヒト”ロチオ-p-トルイシ”ン. 译自PB 85687.抄录如下。
[2-(p-アミノフエニル)-β-メチルヘンソチアソ-ル]: p-トルイシン1240 kg,硫黄445 kg, Na2CO3 2-3 kgを1 hて”1800に上け”,180-1900て”31/2 h, 190-2000て”3-4 h, 200-2100て”3-4 h, 220-2250て”3-4 h热する。发生してH2Sは吸收装置に導いてNaHS 350-380 kgを回收する。
熔融物は蒸馏釜に移し5-10 mmて”釜の温度1500まてて”p-トルイシ”ン480 kgを回收する。つき”に别の蒸馏釜て”4-5 mm小カラムの顺240-2500て”Dehydrothio-p-toluidineを蒸馏し,N2て”ハ”キウムをこわし,1000以下に冷してから碎きとる。残渣50-100 kg.
アルコ-ル500 l.と600に1 h热して冷し,滤過,アルコ-ルて洗い,密闭亁燥する。470-480 kg,收率57%。cp. 190-1920.
张澍声。《精细化工中间体工业生产技术》 1996年。 P. 263. 译自BIOS
1153,349. 抄录如下。
对甲苯胺与硫一起熔融,反应完成后,蒸馏出过量的对甲苯胺,回收的对甲苯胺用于下次合成。粗品脱氢硫代对甲苯胺进行蒸馏。固化后打碎,进行乙醚萃取,醇处理过的产品进行压滤,干燥。所用乙醇蒸馏回收,可复用。
熔融产生的氯化氢通过回收系统,转化为硫氢化钠。
(一)缩合: 将1240 kg对甲苯胺,445 kg 硫和2-3 kg Na2CO3加到熔融锅中,在搅拌下于1小时内加热到1800C。于3.5小时内升温至180-1900C,再于3-4小时内升温至190-2000C,再于3-4小时内升温至200-2100C.在约1800C释出H2S,一直继续至2200C,送往吸收系统。当达到2200C,再于3-4小时内缓缓升至2250C,熔融总时间18-20小时。
(二)回收对甲苯胺: 熔融物抽吸到蒸馏釜中,缓缓抽真空。反应物到达釜内温度为180-2000C,于约3小时降至1200C,釜内真空达到5-10 mm. 对甲苯胺流出。当塔顶温度达到125-1300C,蒸馏完成,釜内温度升至1500C,蒸馏总时间为10小时。回收对甲苯胺480 kg,应用于下次反应,回收占加料的38.7%。
(三)粗品的精馏: 将粗品熔融,抽吸到另一蒸馏釜内,加热到1800C, 真空为4-5 mm。当小塔顶的温度为240-2500C,蒸馏完成。如果真空度差,可高达3000C, 总蒸馏时间10-12小时。有50-100 kg 残渣,用氮气破坏真空,残渣用人工从釜中掏到盘内,作为废物弃去。蒸馏过的脱氢硫代对甲苯胺在接收器中固化,当温度降至1000C以下,打开大盖,进行粉碎,转移到敞口桶中,熔点150-1540C。
(四)乙醚萃取: 在萃取器中加入500 L乙醇,将上述产品加入,在800C回流搅拌1小时,冷却至室温,产品中的杂质因此溶解于乙醇中,离开悬浮的脱氢硫代对甲苯胺。
(五)过滤: 萃取物进行压滤,先用50 L乙醇洗涤,再洗涤2-3次,直至滤液清晰,尽可能压干。
(六)干燥: 上述滤饼用蒸汽加热,蒸出乙醇。得到470-480 kg脱氢硫代对甲苯胺,熔点190-1920C,收率57%。回收350-380 kg NaHS。脱氢硫代对甲苯胺又名2-(4’-氨基苯基)-6-甲基苯并噻唑。
PB 25624, 1122-1135. Verfahren zur Darstellung von Thiotoluidin
(Dehydrothiotoluidin). Mainkur. 1932年12月30日。摘录。
II. Chemischer
Vorgang: 化学过程: 上面反应式是按它抄录的!
In ueberschuessigem p-Toluidin tritt bei
Temperaturen von 16-2250 ansteigend molekularer Schwefel mit p-Toluidin in Reaktion. Es bildet sich
dabei zuerst 3-Merkapto-4-toluidin(抄注:CAS号[23451-96-9]),das
dann mit weiterem p-Toluidin Dehydrothiotoluidin bildet. Die aus dem Kern und der Methylgruppe austretenden 6 Wasserstoffatome
gehen als Schwefelwasserstoff fluechtig:
III. Einsatz: 1237 kg p-Toluidin. 445 kg
Schwefel. 3 kg Soda (zur Neutralisation
das evtl. Schwefels). 1200 kg
Spiritus.
IV. Ausbeute: 640 kg Rohbase. 490 kg
p-Toluidin regeneriert = 39.6% vom Einsatz. 470 kg Thiotoluidin rein Mol 240=56.1% von
Theorie. 1135 kg Spiritus = 94.6% von
Einsatz.
V.
Arbeitswweise: 操作步骤: 以下只抄录一部分,这里不再抄录。读者如果感兴趣,请看德文原件。
PB 25630, 822. PB
73825, 286和 PB 74181, 2945. 德文生产工艺。未抄录。
PB 70188, 6347-6349. 德文分析方法。 Nr. 186. 未抄录。
陈忠源 207年7月12日 于 无锡 明辉国际。













