CAS号 [89-57-6] 生产工艺 5-氨基水杨酸(5-ASA)
CAS名: Benzoic acid, 5-amino-2-hydroxy- 历史参考文献: Beil. 14, 580; E1, 650; E2, 352; E3, 1456.
用途: 医药。染料:酸性绿34。直接蓝148, 149, 159, 162, 163。直接黑48, 49, 51, 97, 122。媒介黄33。 媒介红29。
媒介紫27。 媒介绿12。
媒介棕36。
媒介黑5, 50, 62, 68。反应类别: 偶氮基还原裂解。硝基还原。
生产工艺参考文献: 按本人手头资料整理如下:
BIOS 1153, 244-247.(=胶卷PB 85687)5-Aminosalicylic acid. (Leverkusen)英国人译自德文(未说明来源)。抄录如下。
反应式: 本人有加注。德文原件暂未找到!(有两条路线:偶氮基还原裂解和硝基还原法)
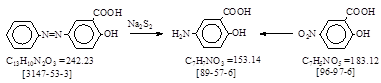
Outline: The aniline à salicylic acid dye is split with sodium
polysulphide.
Plant Details: 略。
Materials: 2200 kg. – 2500
kg. Benzeneazosaicylic acid paste ≈ 230 kg. NaNO2 base on starting salicylic
acid.
460 kg. 60% sodium sulphide. 85 kg. Sulphur. 700 kg. 400Be’ sulphuric acid
600 kg. 190Be’
hydrochloric acid. 25 kg.
Charcoal. 200 kg. Soda ash.
Process:
The 7,000 l. reducer is charged with 4,000 l. of water,
or wash distillate from a previous batch. This is followed by 460 kg. of sodium
sulphide, 85 kg. of sulphur and 1 batch of benzeneazosalicylic acid paste (≈ 230 kg. NaNO2 salicylic acid).
The volume is brought up to 6,500 l. with water and the
temperature raised to 1000C. by means of coil: after heating for 1-2
hours the reflux commence to deliver aniline-water. The amount distillated over
is in the order of 4,300 l. and the rate of distillation is 150-200 l. per
hour. The distillation is continued until all the aniline is off.
Test: A
litre of the distillate when made acid with hydrochloric acid does not absorb
more than 2 g. NaNO2 and a sample from the vessel should not give
any violet colouration with sodium
nitro-prusside. The solution is a dirty green without yellowness.
The volume is now brought up to 6,000 l. and at 600C.
a sample is taken to the laboratory for test. This should provide a yield
equivalent to 85%.
The mass is cooled to 300C. and blown over
into two 7,000 l. tanks. Into the mixture is now run 700 kg. of 50% sulphuric
acid over a long period and with agitation. The liberated H2S is
trapped in dilute caustic soda (1,000 l. of water and 400 kg. of caustic soda).
The reaction is made just acid (Congo brown). The crude amino salicylic acid
being precipitated as a grayish paste.
Test: A
sample is filtered off in the laboratory and the mother liquors heated up with
concentrated hydrochloric acid for 1/2 hour, cooled, and
titrated 1,000 cc. should not absorb more than 2 g. NaNO2.
The batch is then filtered in two portions through a
large press and washed for 10 minutes with water. The H2S trap is
emptied from time to time (after approx. 20 batches of 5-aminosalicylic acid
have been made) and the solution used as starting sodium sulphide for other
batches in small portions.
Purification:
The press cake is charged into an acid resisting
vessel, 9,000 l. capacity, containing 3,000 l. of water, and 600 kg. of
hydrochloric acid are added. The whole is heated with direct steam to 950C.
(Final volume 6,500 l.), treated with about 25 kg. of charcoal, dropped to the
montejus and filtered through a press.
The press is washed with 500 l. of hot water which has
been previously acidified with 20 l. of hydrochloric acid.
Test: 100
g. of the press cake should not absorb more than 0.6 g. NaNO2 after
acidification with hydrochloric acid.
The liquors from the press are returned to 2 9000 l.
vat containing lead cooling-coils, neutralized with soda ash to the Congo brown
stage, about 200 kg. soda being required, and than cooled to 200C.
Test: A
sample is filtered and 1,000 c. should not absorb more than 1.5 to 2.0 g.
nitrite.
The batch is filtered through a tile-lined nutsche, and
washed two to three times with water. The second and third wash can be used as
a basis for the next batch but in general, is not of any value and is best
turned to drain.
Yield: 600 kg. paste (100 g. ≈ 32 g. NaNO2) = 192 kg. NaNO2 = 83.4% theory.
抄注: 水杨酸硝化法,有异构体需要分离,BIOS 1153有报导。
细田豊 《理论制造染料化学》1957年。P. 474. 5-アミノサリチル酸。译自PB
85687. 抄录如下。
サリチル酸NaNO2 230 kg相当とシ”アソ”アニリンをカツフ0リンク”してヘ”ンセ”ンアソ”サリチル酸ヘ0-スト2200-2500 kgを得る。
7 m3 还原釜に水4 t, Na2S 60% 460 kg, S 85 kgおよひ”前记アソヘ-ストを装入し6.5 m3とし,
1000て”12 h加热后アニリン水の蒸馏を始め,每时150-200 lの割て”4.3 m3を蒸馏する。300に冷し7 m3タイル张槽2つに排出し,50% 硫酸700 kgを长时间かけて加え1),酸性になりかけた时(コンコ’-フ”ラウン)に止めて粗制アミノサリチル酸を滤過し少量の水て”洗う。
ケ-クを水3 t + 盐酸600 kgと生蒸汽て”950に上け”,脱色炭25 kgを加え滤過,湯500 l + 盐酸20 lて”洗い,滤液をNa2CO3约200 kgて”コンコ”-フ”ラウ程度に中和し20て”滤過,水洗する。ヘ-スト600 kg(NaNO2 192 kg相当),收率83.4%。
1) H2Sか出るのをNaOH 400 kg + 水1 tに吸收,20 回分位ためてNa2Sとして利用する。
张澍声 编译。《精细化工中间体工业生产技术》 1996年。P. 86. 译自BIOS
1153, 244. 5-氨基水杨酸。抄录如下。
苯胺重氮化,与水杨酸偶合,再用多硫化钠将偶氮基裂解。
(一)粗品合成: 在7000 L铸铁还原锅中,加入4000 L水或上一次反应中蒸馏出的水,随后加入460 kg 60% Na2S, 85 kg 硫磺和2200-2500 kg本偶氮基水杨酸滤饼,相当于230 kg NaNO2,以水杨酸计。
加水使体积达6500 L,升温至1000C, 回流1-2小时后,开始排出苯胺-水,馏出物约为4300 L, 蒸馏速度为150-200 L/ hr,
蒸馏继续到蒸出全部苯胺。
检验; 1 L馏出物用盐酸酸化后,消耗NaNO2 不多于2 g,从锅内取样遇硝普酸钠试纸不应产生紫色,溶液为暗绿色,而不是黄色。
加水使体积达6000 L,在600C取样至实验室试验,收率应达到85%。
物料冷却到300C,送往两个7000 L的槽中,在较长时间内向混合物中流入700 kg 50% H2SO4,并进行搅拌,释出的H2S用稀氢氧化钠溶液(400 kg NaOH在1000 L水中)吸收。溶液应对刚果红为酸性,粗品5-氨基水杨酸沉淀为灰色。
检验: 取样过滤,滤液与浓盐酸加热0.5小时,冷却,滴定,1000 ml样品消耗NaNO2不多于2 g。
反应物过滤,水洗10分钟,得到粗品滤饼,硫化氢洗气塔随时都是空的(约合成20批5-氨基水杨酸后),溶液的小部分用于另一批得原料硫化钠。
(二)精制: 将上述5-氨基水杨酸滤饼加到3000 L水和600 kg 30% 盐酸中,用直接蒸汽加热到950C,最终体积为6500 L,用25 kg活性炭处理。压滤,用500 L热水洗涤,热水预先用20 L盐酸酸化。
检验: 取100 g滤渣,用盐酸酸化后,耗用NaNO2 不多于0.6 g。
上述滤液用碳酸钠中和到对刚果红恰好为棕色,约需200 kg碳酸钠。然后冷却到200C,过滤,水洗2-3次,洗水排放。得到600 kg 5-氨基水杨酸滤饼,相当于192 kg NaNO2,收率83.4%。
俄。A.B.
Eльцова. 染料及中间体实验室合成方法。1985年。§ 7.21 直接重氮灰X。5-氨基水杨酸。译文如下。
2-羟基-5-苯基偶氮苯甲酸: [3147-53-3] 略。
5-氨基水杨酸: [89-57-6]。预先准备: 30 ml 40% 氢氧化钠溶液。
将装有搅拌和温度计的250 ml瓷杯,置于电加热水浴中,固定在卡圈内,加入24 ml 40% 氢氧化钠溶液,2-羟基-5-苯基偶氮苯甲酸滤饼,搅拌下加热到80-850C,分小分加入24
g保险粉(10-20分钟),反应液无色即为还原终点,如仍未变为无色,追加一些保险粉(介质应对酚酞试纸显强碱性)。
反应物变为无色后,保持20分钟,移至500 ml耐热园底蒸馏烧瓶内,蒸出苯胺,5-氨基水杨酸热溶液用布氏漏斗过滤,将装有搅拌和温度计的250 ml瓷杯,置于加热器上,固定在卡圈内,加入热滤液,加热到70-800C,滴加20 ml 27.5%盐酸中和到广泛试纸呈中性,然后滴加浓醋酸酸化到广泛试纸呈弱酸性(pH 4-5),5-氨基水杨酸成结晶析出,悬浮液放置过夜,用布氏漏斗过滤,滤饼抽干,用冷水洗涤,≈20 ml, 置于瓷皿中,于70-750C烘箱中干燥。
得量: ≈8 g (- 85%),熔点2830C; 易溶于水,乙醇,碱;难溶于乙醚。
陈忠源 2017年8月14日 于 无锡 明辉国际。













