CAS号 [108-46-3] 生产工艺 间苯二酚
CAS名: 1,3-Benzenediol 历史参考文献: Beil.
6, 796; E1, 398; E2, 802; E3, 4292; E4, 5658.
用途: 染料:酸性黄73, 74。 酸性橙6, 11, 24, 25。 酸性红51, 87, 91, 92, 95, 98。 酸性绿68。 酸性棕14, 15, 75, 83, 84, 85,92, 97, 105, 113, 119, 121,
122, 123, 138, 143, 144, 213, 214, 216, 233, 235, 354, 367, 417, 418, 440, 452,
453, 469, 470,471, 472。 酸性黑102。 直接橙18, 32, 82。 直接红50, 68, 185。 直接棕5, 6, 18, 21, 89, 95, 100, 119, 123, 148,159, 173,
175, 185, 190。 直接黑174。 食品黄8。 食品橙3。 媒介红5, 59, 67。 溶剂黄174。 颜料红174。
其它:医药,合成树脂等。生产工艺参考文献: 本人按手头资料整理如下:
BIOS 986, 375-376. (=胶卷 PB 77764) No.
181. Resorcinol (Hoechst). 英国人译自德文(未说明资料来源)。抄录如下。
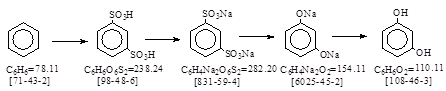
反应式: 本人有加注。第一步是苯磺酸,反应式略,以下含不同原件的英译文,除未说明来源,也未说明其年份!
Process:
1500 kg.
monohydrate in a C.I. vessel with Vortex agitation, 60 r.p.m. Capacity of pan 3 cu.m. The pan was in a water jacket, the water
being heated by steam in jackets. 600
kg. Benzene, thiophene free, E.P. 5.1℃ is run in over 4 hrs. at a temp. of 40℃.
Raised to 100℃.
and maintained for 1/2 hr. Cooled to 50℃ and blown to store.
The disulfonation stage is carried out in a pan exactly
similar and in this pan is charged 2050 kg. Oleum 65%.
The monosulfonation is now run in to the Oleum at such
a rate as to maintain the temp. at 80℃. This takes 7
hrs. and the mass is kept at 80-85℃. for 3 hrs.
Dilute with 4000 l. water and add 500 kg. Sod. Sulfate. Calcium hydroxide is added until neutral and
the mass is filtered on a sand filter and the gypsum washed. The Sod. Salts are formed by the addition of
soda, and the carbonate filtered off, and the liquors evaporated to 400Be’. And
evaporated to dryness on a Venuleth drier.
Fusion of the di-sodium salt: This is done in an electrically heated vessel
with a strong multi-bladed stirrer. The
speed of he agitator was 20 r.p.m. The
weight charge of disulpho is fed to the fusion vessels, which are in duplicate,
by an overhead carrying system.
The charge: 560
kg. caustic soda and 800 kg. Disodium salt are charged alternately whilst
heating to 320-340℃. The fusion is carried on for 8 hr. at 330℃. It
is then run off via a special bottom run-off valve to the dilution vessel which
is immediately below. From here it is
blown to the neutralization vessel (rubber-lined steel tank) and here diluted
to a 1000 gall. Approx and neutralized with HCl. The Sod. Sulfite is filtered off and the
liquor is blown to another vessel. The
sulfite is converted to sod. Thiosulfate and sold.
The liquor is treated with 9 kg. of animal charcoal
and screened and goes forward to be extracted by means of ethyl other.
The extraction is carried out in an agitated pan type
extractor and ether distilled off. The
material is distilled under vacuum.
Yield = 82% theory from Benzene.
Sublimated Resorcine:
In high vac. sublime: 300 kg. : 50 hrs. at 1700. Yield = 230 kg. Output 35 tons Resorcinol.
Technical per month.
Labour: 5 men per 8 hrs. shift.
BIOS 1153, 24-30. (=胶卷 PB 85687) Resorcinol.
(Hoechst). 英国人译自德文(未说明资料来源)。 抄录如下。
The detailed information given here amplifies that
given in BIOS Report No. 986. Flow
diagrams for the sulphonation and fusion stages are given on pages 366 and 367
respectively, and a detailed drawing of the fusion vessel is on page 366. The original drawing of the fusion vessel is
available for reference under the number BIOS/DOCS/2526/1578/45. (抄注: 这是英国人的存档编号)。
Sumary:
The monosulphonation of benzene is carried out with
sulphuric acid 100%, and the disulphonation by running the monosulphonation
mixture into 65% oleum. The mixture is
then run into sodium sulphate solution and limed out. After filtering off the gypsum, sodiation is
completed by the addition of sodium carbonate, the resulting chalk formed being
screened off. The solution is then
concentrated, and dried on a drum drier.
The dried benzene sodium disulpho salt is fused with
caustic flake, and the fusion run into cold water. After cooling the melt solution is made
neutral with hydrochloric acid and the sodium sulphite filtered off; the
solution is acidified, charcoal added, and screened. An other extraction is then carried out, the
other finally being evaporated, leaving crude resorcinol which is distilled
under vacuum.
Process: (a) Disodium
benzenedisulphonate.
Monosulphonation:
苯磺酸 C6H6O3S = 158.18. CAS号[98-11-3]. 苯磺酸钠盐 C6H5NaO3S
=180.16. CAS号[515-42-4]。
600 kg. of benzene are charged to the sulphonator, and
1500 kg. of sulphuric acid 100% run in with agitation over a period of 4 hours
at 50℃. The temperature is then raised at a rate of
16 to 17℃. per hour to 100℃., this step taking 3 hours. On reaching this temperature, the
sulphonation is held for a further hour.
It is cooled to about 50℃.
in 1 hour and blown to a storage tank in 1/2 hour.
Cycle time for monosulphonation = 10 hours.
Disulphonation:
The disulphonation pan is charged with 2050 kg. of 65% oleum in 1/2
hour. This is then warmed to 30℃. in a few minutes, and the addition, with
agitation, of the monosulphonation commenced.
The monosulphonation mass is added over a period of 5 hours, the
temperature in the disulphonation pan rising during this time from 30℃. to 80℃. Heating is
continued at 80-85℃.
for 1 to 2 hours to complete the reaction.
Time = 61/2 to 71/2 hours.
Part Sodiation and Limed out: Half the above solution is then blown into
600 l. of water containing 500 kg. of sodium sulphate (Na2SO4). The mixture is heated to 100℃. and a lime suspension added steadily over
11/2hours, the temperature being maintained at 100 to 105℃.
The lime suspension has a strength of 25% CaO.
The batch is then dropped to a filter during 1/2 to 1
hour. Time = 3 to 4 hours.
Washing of Gypsum:
The gypsum on the nutsche is washed with 500-600 l. of water at 80 to 90℃.
This is repeated 5 or 6 times until the gypsum is free from the benzene
disulpho salt. In all, about 3,000 l. of
hot water are required. When the density
of the wash is 00Be’; the washing is considered complete. The gypsum is then sucked dry and
dumped. Cycle time = 7 to 8 hours.
Completion of Sodiation: The volume of the half batch is now about 7,000
l. is heated to 95℃.
and soda ash added steadily, about 70 kg. required. The resultant calcium carbonate is then
screened off, and returned to the lime out.
Cycle time = 2 to 3 hours.
Concentration and Drying:
The faintly alkaline solution is concentrated (to 400Be’.)
in a Weygand evaporator to 50% of its original volume (i.e. to 3500 l.), and is
then led to a drum drier. About 1100
kg. actual weight of disodium benzenedisulphonate is obtained per half batch
(i.e. from 300 kg. benzene). The second
half of the disulphonation batch is similarly deal with.
100 kg. benzene M.W. 78 gives with good operation 350
kg. disodium benzenedisulphonate actual weight.
Assuming 96% strength (M.W. 282), this is equivalent to 93% theory.
Raw Material Usages:
The following is an actual plant return. 1 tone of disodium benzenedisulphonate, as
free acid M.W. 238, requires:
Benzene 0.283
tones。 H2SO4 0.725 tones。 Oleum
65% 0.985 tones。 Soda 0.065 tones。
Lime as CaO
1.100 tones。 Na2SO4 0.4900 tones。 Yield = 86% theory. This is abnormally low.
Service Usages:
1 tone of benzene disulphonic acid as free acid M.W.
238 requires: Electricity 160 kWh.
Steam 5.5 tones.
Water 165
cu.m. Air 270 cu.m. Man hours process 30.
(b) Resorcinol: Melt:
800 kg. of disulpho salt are charged into the melt
kettle, which has been electrically heated to about 250℃.
The charging of the disulpho takes 20 minutes, and is followed during 10
minutes by 560 kg. of caustic soda flake.
The temperature of the melt is then raised over a period of 6 hours to
340℃. When the reaction is complete. Agitation during the whole series of
operation must be efficient. The melt is
then run to the dissolver containing 500 l. of cold water during 15 to 20
minutes. Time = 63/4 to 7 hours.
Neutralsation and Filteration of Sulphate:
The melt solution is then cooled to 40℃.- 45℃. by circulating water round the pan jacket during 21/2
hours. The solution is then run into
hydrochloric acid 30% until a test on Curcuma paper shows only a faint
colouration. The mixture is now about 60
to 70℃., and the sodium
sulphite is filtered off at this temperature on a nutsche with glass as filter
media. Time = about 5 hours.
Washing the sodium sulphite:
A solution (400-500 l.) of sodium sulphite of 200Be’
is made up with solid sulphite from a previous batch and this at 60 to 70℃. is used for washing the sodium sulphite free
from resorcinol. The washing is done by
running 100 l. of this solution at a time onto the nutsche and sucking
through. This is repeated three or four
times. The solid sulphite is removed for
sale. The solid sulphite should not
retain more than 0.5% of the yield.
Time = 4 to 5 hours.
Acidification:
The solution at 30 to 40℃, is then adidified with hydrochloric acid 30%, and to
the mixture is added 10 kg. of active charcoal.
The volume is then 1,000 l. The
batch is screened through a press, the filter media being vinyl chloride fabric
backed by filter paper, the filter paper being placed next to the wood. The filtration takes half-an-hour. The charcoal in the press is washed with 100
l. of water, the press cleaned and the charcoal discarded. The liquor runs into one of the extraction
vessels in the ether extraction plant.
Ether Extraction:
The solution from 4 batches is collected in the other
extraction plant in a vessel of about 7500 l. capacity. 600 l. of ether are used in the extraction,
the air space in the vessel being made up entirely by the addition of liquor
from a previously extracted batch. The
ether circulates by an overflow from the extraction vessel to an evaporating
pan, the vapour being condensed in a M.S. coil and returned to the extraction
pan. Fresh ether is thus returned
steadily to the extraction vessel, whilst the ether solution of resorcinol
passes to the evaporating pan, where the ether is evaporated off, leaving the
resorcinol. This is done over a period
of 18 hours. Some of the liquor is then
pumped to storage and the ether overflow shut off. The ether is then distilled into the
extraction vessel so that the resorcinol becomes ether free. This part takes 1 hour. The extracted liquor is run to drain from the
storage as required.
The resorcinol is then run into the still during 1/2
hour.
Distillation:
Two batches are worked up together. The distillation is carried out under a
vacuum of 8 mm. on the still body, and the rate of distillation is
approximately 70 kg./hr. The
distillation temperature is 173℃.
rising to 190℃. – 200℃. at the end, the total time of
distillation being about 8 hours. The
first fraction amounting to 2-5 kg., is collected separately and returned to
the next distillation.
The tar formed is discharged from the still after
several distillations by blowing with steam.
This is probably about 1% to 2% of the still charge. The receivers are emptied by running into
cascade beakers, which when cool are emptied, and the material brocken up in a
crusher. From two melt batches 508 kg.
of resorcinol are obtained. Cycle time
= Approximately 12 hours per batch.
Raw Material Consumptions: 1 tone of resorcinol consumes: 单位:吨。苯 0.9。 100% 硫酸 2.2。 65% 发烟硫酸 3.1。
硫酸钠 1.5。 CaO 3.4。 碳酸钠 0.2。 氢氧化钠 2.2。 30%
盐酸 4.3。 乙醚 0.05。 活性炭 0.004。
Recovery: 亚硫酸钠 1.6。
100% 石膏 6.5。
yield from benzene = 78.2% of theory.
(Note: with good operation, a yield of 82-83# can be obtained)
Service Consumptions:
电2700 千瓦小时。 蒸汽 30 吨。 冷却水 1000立方米。 空气 2000立方米。 人工 80小时。
Specification of Final Product: The crystallizing point should be 109.1 to
109.2℃.
FIAT 1313, I, 249-253. (=胶卷 PB 85172) Resorcinol, technical and sublimed. (hoechst)。美国人译自德文。抄录如下。
(This
information is inadequate. It will be more accurately and more completely
covered in BIOS - 1153)
Plant capacity:
35 tons. Equipment: 共 20 台。略。 Materials used per ton: 与BIOS 1153 相同。
By-products produced: 与BIOS 1153 相同。 Service
requirements per ton of product: 与BIOS 1153 相同。
Procedure: 抄注:括号内数字为设备号。译文也未说明资料来源和生产工艺年份。投料量不用kg? 抄录文不再分项!
600 parts by weight of benzene pure flow within 4
hours into 1500 parts by weight of sulfuric acid monohydrate in (1) at about 50℃.
The mixture is then heated up to 100℃ within 3 hours, stirred for 3 hours at this
temperature cooled to about 50℃. 2100 parts by weight of the sulfonation
mixture from (1) slowly run within 5 hours into 2050 parts by weight of oleum
65% in (2) at 30-80℃
while cooling. After stirring for 3/4 hours
at 80℃ half of the
disulfonation mixture is pressed into an aqueous solution of 500 parts by
weight of sodium sulfate in (3) and neutralized with milk of lime containing
about 25% of CaO. After suction of the
calcium sulfate in (4) the solution is mixed in (5) with soda, until calcium
carbonate is precipitated no more. The
solution of the “benzene bisalt” freed from the calcium carbonate by filtration
in (6) is boiled down in (7) and evaporated to dryness on the cylinder
(8). 800 parts by weight of “benzene-bisalt”
and 560 parts by weight of flaked caustic soda are heated in the baking vessel fitted
with stirrer (9) up to 340℃. The baked product is stirred into water in
(10), conveyed to (11) and neutralized with hydrochloric acid. (德文:Benzol bisalz)
The separated sodium sulfite is filtered off in (12),
the filtrate slightly acidified with hydrochloric acid in (13), mixed with some
Esbit carbon and filtered through the filter press (14). The clear resorcinol liquor is then extracted
with ether in (15) and (16). After
complete evaporation of the ether and dehydration in (17) the crude resorcinol
is purified by vacuum distillation in (18).
The liquid distillate is run into enameled cans and allowed to
solidify. The contents of the cans is
discharged and – after shattering of the blocks – the technical resorcinol is
broken into lumps in the jaw crusher (20).
Control: Monosulfonation: 苯一磺化以及后续二磺化,碱熔的生产控制点: BIOS 1153 均未翻译!
1) Examination
for complete sulfonation by smell test after dilution with water。 2) Test for % acidity by titration with n/2
caustic soda solution。
3) Occasional test for % sulfones
by benzene extracting the monosulfonation mixture diluted with water and
weighing residue after evaporation of benzene。
Examination of the calcium sulfate: Examination of the calcium sulfate for
absence of “bisalt” by hydrometer test of the wash liquor.
“Bisalt”: 1)
Determination of the water content。 2) Determination of the soda content by
titration with acid。
Baking melt:
Occasional tests. 1) % alkalinity by titration with n/2 acid。 2) % sulfite content by treatment of the melt
with dilute sulfuric acid and titration of the sulfur dioxide evolved with n/10
iodine solution。
Crude sulfite:
Test for absence of resorcinol by stirring the sulfite with some water,
filtering and adding 30% formaldehyde solution to the solution acidified with
hydrochloric acid; no precipitation of red flakes should result.
Extraction with ether:
Test for complete extraction by extracting a sample from the plant (1 l.
liquor) several times with ether in the laboratory. After removal of the ether the residue must
not amount to more than 0.5 g.
Distillation of the resorcinol in the laboratory: Before starting the plant distillation a
laboratory distillation is always made.
The residue from the distillation must not exceed 8%.
Quality: 原料及成品质量要求: Benzene: solidification point at least 5.1℃. Other
raw materials: commercial quality.
Resorcinol:
solidification point 109.2℃; solution in water and alcohol: clear and colorless; solution
in dilute caustic soda solution:
Clear, slightly yellowish colored.
FIAT 1313, I, 249-250.
Resorcinol, sublimed. 升华级间苯二酚生产工艺。 美国人译自德文(未说明资料来源)抄录如下。
Plant capacity:
2.5 tons. Equipment: 4台,略。 Materials used per ton: 略。 Service
requirements per ton of product: 略。
Procedure:
300 kg. of resorcin techn. Divided into 5 aluminium
troughs are placed on the heating plates of (1). After closing the sublimation apparatus, it
is evacuated and the heating plates are heated with steam. At a vacuum of 0.5 tp 0.7 mm. and a temperature
of 109 to 110℃ in the mass, the
sublimation is finished within about 30 hours.
After cooling and airing of the apparatus the sublimate is taken out
with an aluminium rake and freed from adhering foreign odor on V2A sheets in
(3) at a temperature of 60 to 70℃. The sublimate
is then sieved in (4) and made ready for transportation.
Control: The
product is tested in the Pharmaceutical Research Laboratory for color and odor,
solubility, mechanical impurities etc., according to the demands of the
pharmacopedia in question.
Quality: Resorcin
techn.: commercial quality.
细田豊 《理论制造染料化学》 1957年。 P.
476. 1 レゾルシン. 译自BIOS 1153.抄录如下。
(1)ベンゼン-m-ジスルホン酸: 100% 硫酸1500 kgをベンゼン600 kgに500で4 hかかつて加え,3 hで1000に上げ1 h保温後500に下げて贮槽に排出する。ジスルホン化釜に65% SO3 2050 kgを入れ300でモノスルホン化混合物を入れ始め5
hで终り,800に上る。80-850で1-2 h 加热する。
水1.2 m3 + Na2SO4 1 t に排出し,25% CaO乳液でライミンゲし滤洗する。约14 m3。Na2CO3约140 kgを加え滤過,半分まで浓缩(400Be’)後ドラム亁燥する。ジスルホン酸Na
2.1 t, 纯度96%,收率93%。
(2) Resorcinol: 电气加热式熔融釜を约250に上げジスルホ盐800 kgを装入しつぎにNaOHフレ-ク560 kgを10 mで入れ,6 hで3400に上げ,水500 l に排出する。40-450に冷し,盐酸でNaOH を中和した後60-700でNa2SO3を滤過,Na2SO3 20%液400-500 l で洗い,滤液约1 m3を30-40で盐酸酸性にし活性炭10 kgを加え滤過して抽出に送る。
7.5 m3のエ-テル抽出机に熔融4回分の溶液入れエ-テル600 l で抽出し,エ- テル溶液をオ- バ- フロ- させ,蒸馏して新しいエ- テル抽出に循环する如くし18 h 位で抽出を终り,8 mm 真空で190-2000で蒸馏する。收率ベンゼンから约80%。
Na2SO3 1.6 t / レゾルシン t。
张澍声 《精细化工中间体工业生产技术》 1996年。 P.
153-154. 间苯二酚。 译自FIAT 1313, I, 251; BIOS 986, 375. 抄录如下。
(一)工业品制备: 在2500 L铸铁磺化锅中,在50℃于4小时内将600 kg纯苯加到1500 kg 硫酸一水合物中,然后于3小时内将混合物加热到100℃,在此温度搅拌3小时,冷却到50℃。
在3000 L锻铁磺化锅中预先加入2050份65%发烟硫酸,在30-80℃及搅拌下于5小时内将2100 kg上述磺化混合物缓缓流入,在80℃搅拌45分钟后,将此双磺化物的一半压入到9000 L硬铅搅拌锅(共2只)中,锅中预先加有500 kg 硫酸钠制成的水溶液,然后用约含25%
CaO的石灰乳中和。抽滤除去硫酸钙,滤液与碳酸钠混合,直至不再沉淀出碳酸钙,将苯双磺酸钠的溶液过滤除去碳酸钙,真空蒸发器中蒸发,然后在滚筒干燥机中干燥,得到800 kg 苯双磺酸钠。
将苯双磺酸钠与500份片状氢氧化钠在碱熔锅加热到340℃,碱熔在330℃进行8小时。将碱熔产物搅拌到水中溶解然后中和。过滤分离出亚硫酸钠,滤液用盐酸微酸化,加入9 kg活性炭,过滤。澄清的间苯二酚然后用乙醚萃取。当完全蒸发掉乙醚并脱水后,粗间苯二酚真空蒸馏精制。
液体馏出物流入搪瓷盘中,待其固化后取出,破碎成块状,在颌式粉碎机中粉碎。间苯二酚凝固点109.2℃,溶于水和乙醇应为清晰而无色的,溶于稀氢氧化钠溶液为清晰的微黄色,以苯计,收率82%。
(二)升华; 在15 m3衬铝升华设备中,将300 kg工业品间苯二酚分放在5个铝盘中,铝盘放在加热板上,将升华设备关闭,抽真空并加热加热板。在0.5-0.7 mm真空和109-110℃物料温度下,升华在30小时内完成。
设备冷却和通空气后,用铝耙取出升华物,过筛。升华级间苯二酚主要用于医药。所用纯苯不含噻吩,凝固点5.1℃。
副产的亚硫酸钠转化为硫代硫酸钠出售。
PB 25624, 1346-1348.
Benzol bisalz. 苯磺化成间苯二磺酸钠,CAS号 [831-59-4] 德文生产工艺原件。未抄录、
本胶卷沈阳院有收藏,其编号472-43和32-28,是相同的二个胶卷。本人有手抄的目录。
PB 25624, 1349-1353.
Resorcin. M.W. 110. 间苯二酚。CAS号 [108-46-3] 德文生产工艺研究。未抄录。
PB 70155, 685; 688-696; 700-702. Resorcin.
为不同年份的间苯二酚德文生产工艺原件。未抄录。
国内研究动态:
馏江琼 彭孝军 (大连理工大学)。 间氨基苯酚及间苯二酚合成工艺进展。 [J] 染料工业, 1998, 1,
19-23.摘录如下。
3. 间苯二酚合成工艺及研究进展:
3.1. 磺化 / 碱熔法: 此工艺先以苯和硫酸制成间苯二磺酸,再与苛性钠共熔而成。此工艺需用大量强酸强碱,且产生约三倍量的Na2SO3,废酸处理费用较高。此外,反应中形成的间苯二酚钠盐为高粘度的固形物,在实际操作上也很困难。目前,我国均采用该法合成间苯二酚[19].
3.2. 间二异丙苯氧化 / 分解法: 略。
参考文献: [19] 藤田邦彦,化学工业 1995, 59(8),571-2. [20] – [24] 为3.2. 新工艺的参考文献。未见引用上述文献。
随想: 昨天看了我要上春晚,年轻人的相声,feel so much! One night未眠!
1. 在大学时老师教我们如何査阅Beilstein手册,第四版改用英文,至今未见摘录上述特种文献。 2. 老师教我们如何査阅美国Chemical Abstrat. 它是1907年出版至今。美国人对特种文献,编写了《Bibliography of Scientific and Industrial
Report》,其中1-10卷是关于特种文献的,我至今仍抄有一些产品目录。可惜,未见CA 有one world摘录和报导!这就是编写上网的初衷。
3. Some body说:现在流行抢红包,有谁问钱的多少和来源?你的共享资料,Of cause, No question! No answer! 当然,myself需要考虑到效果,有人Question, 我会立即Answer. 否则是随便上网一些资料,只要我还行,还活着!As good as possible!
陈忠源 2017年11月5日。 Sunday.
俄文Вокресенье (方言:袜子搁在鞋里 – 休息日)













