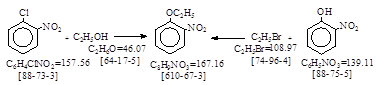
CAS号
[610-67-3] 生产工艺 邻硝基苯乙醚
CAS名:
Benzene, 1-ethoxy-2-nitro- 历史参考文献:
Beil. 未检索。
用途: 香料。染料:如冰染偶合组分等。 反应类别:
见反应式。
BIOS
1153, 16-18. (=胶卷PB
85687)
o-Nitrophenetole. (Leverkusen). 英国人译自德文(无资料来源)
Plant:
略。 Process:
5000 l. of ethanol 94% and 2000 kg, of o-chloronitrobenzene are charged
to the reaction vessel, an air stream of 60 m2 passed through, and the contents
heated to 76℃. Ethanol condensed in the condenser is
returned to the reaction vessel. The
exhaust air is passed through the scrubbing tower and freed from ethanol by
washing with water. The wash from the
tower is ca. 10% ethanol which is later recovered by distillation.
The
dissolving vessel contains 2000 l. of ethanol containing 8-10% of caustic soda,
heated to 35-40℃.
When
the ethanol is refluxing, the ethanol condensate is directed to the caustic
dissolving vessel by means of a 3 way cock, when it dissolves caustic soda and
returns to the reaction vessel. When the
caustic soda content has fallen to below 6%, after 2-4 hours, a fresh charges
of caustic soda (ca. 200 kg.) is charged to the solution vessel. Altogether 820 kg. of caustic soda are required
for the reaction. The ethanol reflux is
returned to the reaction vessel during the caustic addition.
The
temperature in the reaction vessel gradually rises to a maximum of 78℃. The temperature is not allowed to rise above
this (by controlling the rate of caustic soda addition, and also the steam
flow) other-wise side reactions occur (formation of azoxy-compounds) and the
product is worthless.
The
pressure in the vessel during the preparation of o-nitrophenetole from
o-chloronitrobenzene, is not allowed to rise above 66-80 mm./Hg.
The
rate of caustic soda / ethanol addition is controlled by titration of the
reation mass; the alkalinity must not exceed 2% caustic soda.
Agitation
must in on case be allowed to stop and before starting a batch the driving belt
is teated for its trustworthiness. When
reaction is complete the charge must be blown to the autoclave as soon as
possible. It is cooled to 30℃. the blow-leg fitted and blown to the autoclave.
The
charge is neutralized with 150-170 l. of sulphuric acid 78% (280-290 kg.), 200
kg. of soda ash are added, the autoclave is sealed, and 250 kg. of ethyl
chloride are then blown in.
The
autoclave is heated by means of steam on the coil to 100℃.
during 3 hours and raised to 130℃. during a further 2 hours and maintained at 130℃. for 8
hours. The pressure in the autoclave is
10-12 atm.
Isolation: The completed batch is cooled and allowed to
settle. The supernatant liquid is blown
through an enclosed filter and the filtrates collected in an agitated
vessel. The residue of NaCl, Na2SO4, Na2CO3
and sodium nitrophenate is washed 3-4 times with approx. 1000 l. of ethanol,
each wash at 50-60℃.,
and washes blown through the filter.
The
salt residues are dissolved inwater, ethanol recovered by distillation and the
aqueous residue blown to drain. (这就是排入下水道!)。
The
filtered ethanol solution is distilled with direct steam to recover the ethanol
(pressure not more than 120 mm./Hg) and after cooling, the oil is separated
from the water. The oil is washed twice
with dilute NaOH, followed by water. The
washed oil is dried under vacuum and is then again filtered.
Reaction
times: 略。 Yield: 1960 kg. C.P. 4℃. = 92%
of theory. Organic chlorine content: Not
more than 0.2-0.3%.
细田豊
《理论制造染料化学》1957年。 P. 481. o-ニトロフェネト-
ル. 译自PB 85687. 不再抄录。
张澍声
《精细化工中间体工业生产技术》
1996年。P.
71-72. 邻硝基苯乙醚。
译自BIOS
1153, 16. 不再抄录。
国内研究动态 :
袁云程 高大彬 冯苏宁
(大工)。 相转移催化制备邻硝基苯乙醚。
[J] 染料工业,
1991, 2, 14.
实验部分: 取邻硝基苯酚35 g,溴乙烷41
g,氢氧化钠11
g,苄基三甲基氯化铵3
g,水50 ml,加入三口瓶内。搅拌,升温至80℃开始反应,3小时后停止反应。产物分两层,上层是有机层,下层为水层。分离,减压蒸馏得邻硝基苯乙醚,收率94.8%,纯度99.9%,沸程121-124℃/5mmHg.
一位已经Out 了的老人。抄录历史文献,是献给年轻人了解产品历史而已,至少你在图书馆已不可能看到这类资料了!
陈忠源 2018年2月7日。













