CAS号 [101-16-6] 生产工艺。 3-甲氧基二苯胺
CAS名:Benzenamine, 3-methoxy-N-phenyl- 历史参考文献:Beil. 13. 411; E3, 945; E4, 972.
用途:冰染重氮组分47。 有机合成(分散染料。电子化工产品)。 LookChem网登录生产与经营单位60家。
BIOS 986, 399.
(=胶卷PB 77764)。No. 200. 3-Methoxydiphenylamine (I.G. Hoechst ). 英国人抄录,只有反应式。
反应式:本人有加注。(有BASF和 Hoechst 二家厂的生产工艺)。
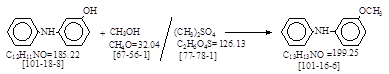
BIOS 1149, 68-69.
3-Methoxydiphenylamine. (Ludwigshafen). 英国人译自德文(无资料来源)。这是BASF 生产工艺。
Plant: 1. Agitated C.I. vessel, jacked and fitted with
reflux condenser. Capacity 1.5
cu.m. 2. 1 Syonr nutsche with iron receiver.
Process:
120 kg. of
methanol are charged into (1) followed by 185 kg. of 3-hydroxydiphenylamine
(small lumps) and the whols stirred at 600 until solution is complete. 2.5 kg. of sodium hydrosulphite are added
followed by 140 l. of 33% caustic soda liquor.
Then 186 kg. of dimethylsulfate are run in at such a rate that the
methanol is kept refluxing without any external heating. This takes about 4 hours. The mixture is stirred for a further hour and
tested for completion of reaction (see below).
When
complete, the product is granulated by the addition of 500 l. of water at 600
and the suspension then cooled to room temperature and filtered on (2). The filter cake is washed twice with 200 l.
of water. Yield = 180 kg. = 90.5%
theory.
Test: 1. The methylation mixture must remain alkaline
throught.
2.
When methylation is complete, the filtrate from a sample diluted with
water should give no precipitate on acidification.
Specification: 3-Hydroxydiphenylamine: M.P. 77℃.
3-Methoxydiphenylamine: M.P. 68℃. B.P.
180℃./5 mm. Purity (by nitrite estimation) 85-90%.
Services
requirements: Electricity 250 Kw/hr. per tone. Steam
0.5 tones per tone.
Water 300 cu.m. per tone.
FIAT 1313, I,
193-194. 3-Methoxy-diphenylamin (I.G. Höechst) “For Blue Base FG” 美国人译自德文(无资料来源)。
Plant Capacity: 4 tons.
Equipmentt: 1) 1
iron stirring vessel with a heating jacket and reflux condenser, capacity 1.5
cu.m.
2)
1 suction filter of stone with an iron receiver for the filtrate.
Materials
used per ton: m-Hydroxy
diphenylamine 1020 kg. Methanol
665 kg. Dimethyl sulfate
crude 1040 kg.
Caustic soda solution
33% 1060 kg. Sodium hydrosulfite 14 kg.
Procedure:
Within (1) 185 kg. of m-hydroxy diphenylamine
in small “crumbs” are introduced in 120 kg. of methanol. The mixture is heated to 60℃. When
complete
solution is obtained 2.5 kg. of sodium hydrosulfite and 140 l. of caustic soda
solution 33# are added. Then 186 kg. of
crude dimethylsulfate are
introduced at
such a rate that the alcohol in kept boiling without any extra heating. The reaction takes about 4 hours. The mixture is stirred for another
hour and is
tested for completeness of the reaction.
The product is granulated with about 500 l. of water at about 60℃, cooled down to room temperature
and filtered
on (2). The product on the suction
filter is covered twice with 200 l. of water each time.
Yield: 180 kg. of a product with a degree
of purity of 100%.
Control: a)
m-Hydroxy diphenylamine: Determination
of the melting point.
b)
During methylation the reaction must be distinctly alkaline to
phenolphthalein.
c)
When a sample of the filtrate is acidified on more starting material
should precipitate.
d)
m-Methoxy diphenylamine:
Determination of the melting point.
Determination of the degree of purity by treatment with nitrous acid.
Quality: m-Hydroxy diphenylamine: M.P. 77℃.
m-Methoxy diphenylamine: M.P. 68℃. B.P.
at 5 mm 180℃. Degree of purity 85-90℃.
FIAT 1313,
III, 143-145. Laboratory Process. 3-Methoxy-Diphenylamine. 译自德文(无资料来源)。
185 g. (Equivalent to 1 mol) of
3-hydroxy-diphenylamine (Ludwigshafen) is dissolved in 500 cc. of alcohol
denatured by ordinary
means. To this solution 200 cc. of potassium
hydroxide 350Be’ which equals 86.4 g.
(1.5 mols) is added and the reaction mixture is
heated to the
boil under very good agitation. To the
boiling solution is added carefully 190 g. (1.5 mols) dimethylsulfate
pure. The
reaction is
violent. After all of the
dimethylsulfate has been added, the reaction mixture is held one hour at the
boil, then is cooled
with
agitation and further cooled to 0℃.
There results a yellow crystalline mass
which is filtered, washed neutral with ice water and dried at 50-60℃. The
product
comprises 202
g. of 3-methoxy-diphenylamine of 96.1% purity which equals 194 g. which equals 97%
of theory. The remaining 3.9%
consist of
N-methyl-3-methoxy-diphenylamine and some moisture.
张澍声 《精细化工中间体工业生产技术》 1996年。P. 26.
3-甲氧基二苯胺。译自BIOS 1149,
65.
在1500 L铸铁锅中加入120 kg甲醇和185 kg 3-羟基二苯胺,在60℃搅拌至完全溶解。加入2.5 kg NaHSO4(抄注:应为保险粉)和140 L 33%
氢氧化钠溶液。然后加入186 kg硫酸二甲酯,加入速度要在没有外部加热下保持回流状态。这约需4小时,混合物再搅拌1小时。整个甲基化过程
必须保持碱性;甲基化完成后,取样过滤,滤液用水稀释,酸化时应不产生沉淀。
甲基化完成,产品加入500 L 60℃的水进行造粒,悬浮体冷却至室温。过滤,滤饼用200 L水洗涤两次。
得到180 kg 100% 3-甲氧基二苯胺,收率90.5%。熔点63℃,沸点180℃ / 5 mm, 纯度85-90%。
PB 73377, 2407-2407. m-Methoxy diphenylamine. 未抄录。
国内研究动态:
大连工学院: 凡拉明蓝FG色基之试制, [J]
有机化学工业技术报导, 1958, 11, 60-61.
试验是按照BIOS 1149 及FIAT 1313第三卷的资料复制的,所以[101-16-6]合成工艺,这里不再抄录。
国内专利:
曹维孝 曹维伟 冯新德 (北京大学)。 中国专利 CN 1034711.
3-甲氧基二苯胺-4-重氮盐的制备方法。
反应式:本人有加注。(3-甲氧基二苯胺的合成方法。)。
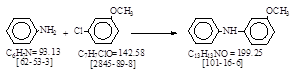
于500毫升三口烧瓶中加入167毫升苯胺,于氮气保护下,将3.7克(0.09摩尔)金属钾加入其中,缓慢加热回流至钾完全消失,随后滴入
9.5克(0.07摩尔)间氯苯甲醚,并保持回流40分钟,将反应物冷却至40℃,慢慢加入100毫升水,再加入30毫升乙醚,分出水相,有机相用5%
盐酸洗至呈酸性,再用水洗至中性,蒸去乙醚和低沸点物后,减压蒸出产品,收集145-148 / 0.5 mmHg馏份,为灰白色固体,用乙酸乙酯-正己烷重
结晶,得3-甲氧基二苯胺,为灰白色晶体,重8克(产率60%),熔点67-68℃。
抄注:无参考资料!也未提及上面的历史资料。(专利中只提到未见有3-甲氧基二苯胺-4-重氮盐的制备方法。)。
学习与思考:
以上是德国二家厂的生产工艺,基本相同,暂未找到德文原件,同时本人也未见到国内手册有报导,只有[J] 有机化学工
业技术报导有试制报导,所以中国专利中说没有参考文献,而所用的合成方法是苯胺与间氯苯甲醚的缩合方法。
历史资料是否有用,请读者自己看吧!
工欲善其事必先利其器:
工匠要把活儿做好,首先要使工具精良。
陈忠源 2018年6月16日星期六。













