CAS号 [1008-72-6] 生产工艺。 2-甲酰基苯磺酸钠
CAS名:Benzenesulfonic acid, 2-formyl-, sodium
salt(1:1). 历史参考文献:待检索。
用途:酸性红289。 酸性蓝2, 2:1。 媒介蓝3。 颜料蓝24, 78.
增白剂351。 医药等。 LookChem网登录单位87家。
BIOS 1153, 314-316.
(=胶卷PB 85687)。 Benzaldehyde-o-sulphonic acid. (Hoechst). 英国人译自德文(无资料来源)。
反应式:本人有加注,德文原件见后(未抄录)。
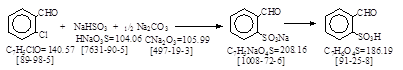
Plant: 设备:共10项,略。
Procedure: 操作步骤:
275 kg. of
soda and approx. 1000 l. of water are heated to the boil in the 7000 l. iron
kettle, and 521 kg. (as 100%) of approximately 52% bisulphite is added from the
measure vessel. The solution is again
raised to the boil, the volume adjusted to 3100 l., 2-3 kg. of soda added to
give a weakly alkaline test on phenol phthalein, and is then blown over with
nitrogen, while still boiling, into the autoclave prewarmed to 90℃.
During these
operation, two 300 kg. drums of o-chlorobenzaldehyde are melted in the hot
water tank and then blown by nitrogen pressure (o.5 atm.) into the hot neutral
sulphite solution contained in the autoclave.
(Water seal as excess pressure valve).
The autoclave is now heated with H.P. steam (25 atm.) at first with open
valves so that the remaining CO2 can escape.
At 100℃. (inside
temperature) the valves are shut, the temperature raised to 190-195℃. during approx. 6 hours, held for 3 hours
and the steam then turned off. The
temperature falls back to approx. 170℃. during 4 hours whereupon the large
needlevalve is slowly opened and the temperature falls to approx. 100℃. in a further 6 hours. The autoclave contents are now blown (approx.
3300 l. of solution) through the screening press into the evaporator.
The clarified
solution is evaporated to 2000 l. and blown while still hot into the crystallizer. The batch is self-cooled to 60℃. and then with cold water (or brine in
summer) to 30℃.
9 l. of 37%
caustic soda liquor is added under which conditions crystallization of product
is complete without too much separation of salt (previously two crystallizations
were carried out with an intermediate removal of salt).
Finally the
batch is cooled to 15℃. and run down to the centrifuge.
The centrifuged, still moist crystalline product is discharged to casks
(or drums) weighed and analysed. The
mother liquor is discharged to the drain.
Yield:
Approx. 850 kg. at 77.6% = 660 kg. at 100% M.W. 186. = approx. 110% by weight
on o-chlorobenzaldehyde. = 83.0% of theory.
FIAT 1313, I,
57.(=胶卷PB 85172) Benzaldehyde o-sulfonic acid. (I.G. Hoechst). 美国人译自德文(无资料来源)。美国人是摘译!
Procedure: Dissolve
275 kg. sodium carbonate in 1000 l. boiling water. To the solution add 521 kg. sodium bisylfite
100% as 52% (approx.) solution. Heat to boil and dilute to 3100 l. Make weakly alkaline to phenolphthalein
papers with 2-3 kg. sodium carbonate.
Blow with nitrogrn into the iron autoclave previously heated to 90℃.
Add 600 kg.
ortho chloro benzaldehyde. During 6
hrs., heat to 190-195℃. At about 100℃., release the pressure (largely carbon
dioxide) on the autoclave. Maintain at
190-195℃. for 3
hours.
Allow the
autoclave to cool back to 170℃. during about 4 hours. Then by
cracking the needle release valve, cool during about 6 hours to 100℃.
Filter the solution (about 3300 l. volume) through filter press into an
iron evaporator. Evaporate to volume of
2000 l.
Transfer
charge to the crystallizer. Allow
temperature to fall by itself to 60℃. Then
cool externally to 30℃. Add 9 l. of 37% sodium
hydroxide to promote crystallization.
Cool to 15℃. Separate the ortho sulfo
benzaldehyde by centrifuge, discarding the mother liquors.
The yield is
850 kg. paste = 660 kg. 100% (Mol wt. 186) = 83.0% of theoretical yield.
细田豊 《理论制造染料化学》 1957年。 P. 501.
Benzaldehyde-o-sulfonic acid. 译自FIAT 1313. No. 36.
Na2CO3 275 kgを湯1 m3に溶し,NaHSO3 521 kgを约52% 液として加え煮沸し,3.1 m3にうすめNa2CO3 2-3 kgを加えてフェノ- ルフタレイン弱アルカリ性としてオ- トクレ- ブに装入する。
o-クロルベンズアルデヒド600 kgを加え,6 hで190-1950に上げ3 h保温する。この间约1000の时CO2をぬく。
1000で滤過し,滤液を3 m3まで蒸发,结晶机に移して300まで冷し,结晶を促进させるために37% NaOH液9 l を加え150に冷し滤過する。ペ- スト850 kg = 660 kg 100%(分子量186)收率83%。
张澍声 《精细化工中间体工业生产技术》 1996年。 P. 120. 苯甲醛邻磺酸。 译自BIOS 1153, 314和FIAT 1313,I, 57.
在7000 L铸铁锅中加入275 kg碳酸钠和约1000 L水,加热至沸,加入1002 kg 52% 亚硫酸氢钠溶液。溶液再升温至沸,体积调节至3100 L,加2-3 kg碳酸钠使酚酞为弱碱性。然后在沸腾下用氮气压送至预热至90℃的高压釜中。
600 kg邻氯苯甲醛在热水槽中熔融,用0.5巴氮气压入含有中性亚硫酸氢钠的高压釜中。高压釜用25巴高压蒸汽加热,起初打开阀门,残留的CO2能够逸出,在内温达100℃时,关闭阀门。于约6小时内温升至190-195℃。在此温度保持3小时,然后关闭高压蒸汽。于4小时内温降至170℃,这时缓缓打开大的针型阀,在6小时内温度降至约100℃。釜内物约为3300 L溶液,压入过滤机,滤液进入蒸发器。该澄清溶液煮沸浓缩至2000 L,趁热送入结晶器。自身冷却到60℃,冷水(或冰盐水)冷却到30℃。
加9 L 37% 氢氧化钠溶液,在此条件下产品的结晶是完全的,无需太多地分离出盐(起初两次结晶要在中间阶段进行除盐)。最后反应物冷却到15℃,流入离心机,离心出来的湿结晶产品装桶,称重并分析。滤液排放。得到约850 kg 77.6%产品,相当于660 kg 100% 产品,收率84%。
PB 25623, 340-345. o-Sulfobemzaldehyde. 德文生产工艺原件。 未抄录(含生产工艺年份)。
PB 25628, 3873-3876. o-Sulfobenzaldehyde. (General aniline works).
1938年10月。英文生产工艺。 未抄录。1美元。
美国人介绍:The
production of this substance from o-chlorobenzaldehyde, sodium carbonate and
sodium bisulfite, is described in detail, including purification. It is stated that so far only small
quantities have been made. And modifications may be necessary. In English.
PB 73726, 432-436. Benzaldehyd-2-sulfosäure. 美国人介绍是分析方法。 1.5美元。 未抄录。
国内研究动态:
解 澈。 邻甲酰基苯磺酸的合成。 [J] 化学世界。1990, 34 (12), 546-547. 未摘录。
邓 刚 林原斌 刘飞孟 黄孝华 (湘潭大学化学学院)。 2-甲酰基苯磺酸钠的合成。 [J] 染料工业, 2002, 5, 37-38.
1.3 2-甲酰基苯磺酸钠的合成:
将75.6 g 亚硫酸钠溶液于330 ml水中,用硫酸调pH值至中性,加入到500 ml高压釜中,再加入28.2 g邻氯苯甲醛和6.2 g亚硫酸氢钠。加热到180℃,釜内压力在1.1 Mpa 左右。反应8小时后,冷却,将反应液倒入500 ml园底单口烧瓶中,减压蒸馏至干。加入300 ml 无水乙醇,加热回流30分钟后,趁热过滤,滤液中有白色固体析出,冷至室温,过滤,烘干,得32.5 g (75.8%) 2-甲酰基苯磺酸钠,白色粉末状固体。将钠盐酸化得到磺酸,为针状晶体,熔点114.3℃ – 116.2℃.,文献值114.1℃ – 115.4℃。
参考文献:10篇。(抄注:未见引用上述历史生产工艺文献!)。
国内出版物:
侯乐山 主编 《中国精细化工产品集 – 原料及中间体 10396种》 2006年。 P. 795. 邻磺酸苯甲醛。
中国化工信息中心 全国精细化工原料及中间体行业协作组 出版。
英文名:o-Sulfonic
acid benzaldehyde. 物质登记状态:未登记。 结构式:略。 生产厂:温州市东亚染料化学品有限公司。
抄注:再无其他资料(说明国内无人整理国内产品的文献资料,国外历史文献已过时?更无人整理!)。
陈忠源 2018年8月7日。













