C.I. 分散蓝3(C.I. 61505)生产工艺。 CAS号 [2475-46-9]
CAS名:9,10-Anthracenedione, 1-[(2-hydroxyethyl)amino]-4-(methylamino)- 历史参考文献:待检索。
用途:各类织物染色。LookChem网登录生产与经营单位38家。 反应类别:缩合。
原版“Colour Index”: 发明者:K. Köberle, R. Schweizer, C. Steigerwald, E.
Runne, and L. Berlin 1933年。
生产工艺参考文献:BIOS 987, 155; BIOS 1484, 57; FIAT 1313,2,
206. FIAT 764 – Cellitonechtblau FFR.
BIOS 987, 155.(=胶卷PB 75860)。No. 1053. 1936年5月19日。Dr. A. Krause. 英国人抄录。
It was shown analytically that the dyestuff Celliton Fast Blue FFR does
not correspond to the formula(见后,CAS号 [2475-46-9])but to a mixture of
1,4-dihydroxyethylaminoanthraquinone, 1,4-dimethylaminoanthraquione with the
above compound [2475-46-9]. Such a mixture
is appreciably stronger tinctorially than its components alone.
BIOS 1484, 57-58.(=胶卷PB 86139)。 Celliton Fast Blue
FFR Powder (Ludwigshafen). 英国人译自德文(无资料来源)。
反应式:本人有加注。《染料品种大全》p. 905. C.I. 分散蓝53. CAS号 [86722-66-9]。
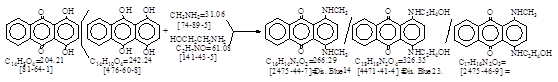
(Mainly 1-methylamino-4-β-hydroxyethylaminoanthraquinone)
An iron pressure vessel is charged with – 1562 kg. isobutanol
containing less than 2% water., 252 kg.
quinizarin sublimed 95%. M.P. 193-195℃.; 63 kg. leucoquinizarin 95%. M.P. 253-255℃.; 91 kg. ethanolamine.; 235 kg. of an isobutanol solution of
methylamine containing 47 kg. 100% methylamine.
The batch is heated in 1-2 hours to 60℃., stirred at 60℃. for 3 hours, then taken in 2-3 hours to the
boil and refluxed for 3 hours. It is now
tested by stirring a few drops with a 1% solution of boric acid in acetic
anhydride. The colour of the solution
should be greenish blue with grey-green fluorescence, but if much quinizarin is
present the colour will be reddish blue with a yellow-brown fluorescence. Smaller amounts of quinizarin can be detected
by spectroscopic examination, the appearance of lines at 470 and 510 being due
to qunizarin. There should be only the
dyestuff lines at 560, 600 and 660.
When a satisfactory test has been obtained the vessel contents are
cooled to 95℃. and 37.5 kg. piperidine and 6.25 kg. of
basic copper acetate are added. Then
heated again to the boil and air blown through at the rate of 2.2 cu. m. per
hour until the oxidation is complete. Test:
A drop of the melt in a large quantity of cold methanol is compared with a
sample of type. The colour should not be
duller. A solution of 1% boric-acetic
anhydride should give a spectrum of 560, 600 and 660, and the solution should
have grey-green fluorescence.
The batch is cooled down, blown on to the nutsch, filtered and washed
with 900 kg. isobutanol. The filter cake
is dried in small quantities under vacuum at 70-80℃. in a Venuleth. It is
necessary to dry the dyestuff as rapidly as possible without exceeding this
temperature, as it loses strength by overheating, and also if the drying takes
a long time there is a loss in colour strength.
Yield = 330 kg.
The dyestuff contains about 5% of another blue substance of unknown
constitution (see. However, BIOS Final Report 987, page 155).
FIAT 1313,II, 206-207.(=胶卷PB 85172)。 Celliton Fast Blue
FFR. (Ludwigshafen). 美国人译自德文(无资料来源)。
Celliton Fast Blue FFR has the above empirical constitution
essentially but is actually a mixture of 1,4-dimethyamino anthraquinone, 1,4-di(oxyethylamino)
anthraquinone and the above.
The process: To 3 cbm. iron kettle add 1562 kg. isobutanol, 252 kg.
quinizarin sublimed 95%, 63 kg. leuco 1,4-diaminoanthraquinone 95%, 91 kg. hydroxyl
ethylamine 95% and 235 kg. of a 20% solution of methylamine in isobutanol. (抄注:这里用的是1,4-二氨基蒽醌隐色体!与BIOS译文有差别!)
Heat to 60℃. during 1-2 hours and hold for 2 hours. Then in 2-3 hours heat to the boil (98-990)
and boil 3 hours. Cool to 95℃ and add 37.5 kg. piperidine and 6.25 kg. basic copper acetate powder. Heat to boil again and blow air through the
charge at 2.2 cbm. per hour about 30 hours until test (color of dissolved
sample) is similar to standard.
Cool to 20-22℃ (24 hours in summer; 15 hours in winter) and
filter in pressure nutsch. Wash with 900
kg. isobutanol from the kettle. The cake
is very voluminous and doesn’t filter too well.
Dry product in Venuleth at 600 mm vacuum with 70-80℃ water on jacket. The yield was
330 kg. = 300 kg. 100% of theoretical.
The isobutanol used contained less than 3% water. Incomplete oxidation caused redder
shades. Higher temperatures or long time
drying harmed the product. This color
was not acid pasted but was converted directly to the dispersible powder.
细田豊 《理论制造染料化学》 1957年。 P. 690. セリトン ファスト ブル- FFR. 译自FIAT 1313.
3 m铁釜にイソブタノ- ル1562 kg,升华キニザリン252 kg (95%),リウコ-1,4-ジアミノアントラキノン(95%)63 kg,ヒドロキシエチルアミン(95%)91 kg,メチルアミン20% イソブブタノ- ル溶液235 kgを装入,1-2 hに600に上げ2 h 保温,2-3 h で沸点(98-990)まで上げ3 h煮沸,950に冷し,ピペリジン37.5 kg + 盐基性酢酸铜6.25 kgを加え再び煮沸,空气を2.2 m3/h で约30 h吹入み,20-220に冷し滤過,イソブタノ- ル900 kgで洗い,Venulethで600 mm,70-800の水通して亁燥する。330 kg = 300 kg 100%,收率81%。硫酸处理は行わない。
抄注:日文译自FIAT,所以也是说:1,4-二氨基蒽醌隐色体。
张澍声 《精细化工中间体及产品生产工艺》2006年. P.
649. 1-甲氨基-4-β-羟乙基氨基蒽醌。 译自FIAT 1313,II, 206.
在3000 L铁锅中,加入1562 kg 异丁醇,252 kg升华的95% 1,4-二羟基蒽醌,63 kg 95% 1,4-二氨基蒽醌隐色体(抄注:CAS号 [5327-72-0]),91 kg 95% 乙醇胺和235 kg 甲胺在异丁醇中的20% 溶液。 于1-2小时内加热到60℃,在60℃保持2小时。然后于2-3小时内加热至沸(98-99℃),并沸腾3小时。冷却到95℃,加入37.5 kg 哌啶和6.25 kg碱式醋酸铜粉末。再加热至沸,向反应物中吹空气30小时,速度2200 L/hr,直至检验满意。即取样溶解后于标准品进行比色。冷却到20-22℃,夏季24小时。冬季15小时。抽滤,用900 kg 异丁醇洗锅。滤饼体积很大,不易过滤好。产品在耙式干燥器中,于600 mmHg及70-80℃干燥。
得到330 kg 1-甲氨基-4-β-羟乙基氨基蒽醌,相当于300 kg 100% 纯品。收率81%。所用异丁醇含水量应不少于3%。不完全氧化使染料色光稍红,较高温度和较长时间干燥对产品有害。
德文原件:
PB 25628, 4147-4155. Cellitonechtblau
FFR powder. 1934年5月德文生产工艺。 1美元。美国人介绍如下,本人未抄录。
Cellitonechtblau FFR
(1-(2-hydroxyethyl)amino)-4-methylamino-anthraquinone) is produced according to
the following reactions: “Leukamin” (1,4-diamino-9,10-anthradiol) reacts with 1
mol ethanolamine and 1 mol methylamine to form the leuco dye (1-(2-hydroxyethylamino)-4-methylamino-9,10-
-anthradiol) and ammonia. 1 mol
leuco dye reduces 1 mol qunizarin (1,4-leuco dye is converted into the dye
(Cellitonechtbalu FFR). 1 mol
leucoquinizarin (1,4-dihydroxy-9,10-anthradiol) reacts with 1 mol ethanolamine
and with 1 mol methylamine to form the leuco dye. The leuco dye reduces quinizarin and is
oxidezed to the dye. The works instructions
are given in great detail and the reaction is exhaustively described. The control tests for every step are
indicated with the greatest details. The
experiences gained and sources of mistakes and troubles are pointed out. (请注意后面这一句!)。In German.
PB 73726, 316-324. Cellitonechtblau FFR = 1-oxäthylamino-4-methylaminoanthraquinon.
1.5美元。
By Hensle and Ehrhardt. 1936年3月4日德文生产工艺。 本人未抄录。
俄。A.B.Eльцова。 《染料及中间体实验室合成方法》。 1958年。§5.2 分散蓝K。本人以前的译文,仅供参考。
在装有搅拌和温度计的100毫升钢压热器中,加入40毫升甲醇,2.1克保险粉,搅拌均匀后加入7.2克1,4-二羟基蒽醌,然后再加入3.6克80% 乙醇胺(d=1.03)和4克甲胺溶液(d=0.97),密闭压热器,慢慢(1-1.5小时)加热至94-95℃,在此温度保温反应3小时,冷却至室温,放压,打开压热器,加入2.36克醋酸铜,搅拌15-20分钟。
250毫升浮氏蒸馏烧瓶,置于加热水浴中,蒸馏瓶引出管与水抽相接,瓶口盖以配有玻璃管的瓶塞,将玻璃管伸到瓶底,另一端接上內装1/3粒状苛性钾的气体干燥瓶,蒸馏烧瓶内加入反应完的物料,加热至65-75℃,在此温度下,4小时内用水抽引入空气,氧化完毕蒸馏烧瓶上装以直管冷凝器,减压蒸馏至近干。瓶内物料用60毫升水稀释,用布氏漏斗过滤,染料滤饼用冷水(每次20毫升,总量100毫升)洗至广泛试纸呈中性,抽干,置于培养皿中,于80-90℃干燥。
得量:8.3克(92%)。加热至300℃不熔。蒲层展开(丙酮:己烷= 1:2,溶剂:丙酮)。Rf 0.63和Rf 0.1 (痕迹量)。
国内研究动态:
沈阳化工研究院分散染料组 《聚酰胺用分散染料几个品种的试制》[J] 化工技术资料 – 染料及中间体专业分册。1965, 2, 10-11.
分散蓝FFR (C.I. Disperse Blue 3. 61605):
将1,4-二羟基蒽醌,甲胺,乙醇胺和锌粉在丁醇溶剂中,先加热反应2小时,使部分1,4-二羟基蒽醌还原成1,4-二羟基蒽醌隐色体,然后逐渐升温到94-97℃,并在94-97℃进行缩合反应,保温8小时,缩合反应就完成。缩合物在搅拌下缓慢冷却至15℃,染料就全部析出。染料浆液经过滤除去丁醇,经水洗,干燥,粉碎后,就得到分散蓝FFR,其收率为70-72%。
参考文献:沈阳化工研究院分散蓝FFR总结报告。参考文献共13篇。未提及本人上面抄录的德文生产工艺。
抄注:研究报告参考的可能是BIOS 1484. 所以没有提到1,4-二氨基蒽醌隐色体。
国内染料专业出版物:
肖 刚 杨新玮 等 主编。全国染料工业信息中心出版 《世界染料品种 – 2005年》。P. 577. C.I. 分散蓝3.
参考文献:只是原版“Colour Index”的译文。1965年的国内研究报告也未提及!
抄注:没有利用以前的,早已进口的历史资料和国内自己发表的资料?在这以前已提过意见,可是人家不信!
何岩彬 主编 《染料品种大全》沈阳出版社 出版 2018年. P.
897. C.I. 分散蓝3.
参考文献:增加部分为本人提供。
这里要再次谢谢何岩彬同志。当然新版写上的PB报告不可能让大家去看,其实今天要再看已不太可能了。
老有所养,自得其乐!!!
提出不成熟的意见,只是希望大家理解,因为老了无事可干,至少没有坏话,也没有不可告人意见和想法。
陈忠源 2019年3月24日星期日。













