CAS号 [16670-48-7] 生产工艺。 苯磺酸氯乙酯
CAS名:Ethanol, 2-chloro-, 1-benzenesulfonate 历史参考文献:Beil. 11. E2, 20; E4, 30.
用途:有机合成。LookChem网登录生产与经营单位10家。 反应类别:脱盐酸缩合。
FIAT 1313, I, 101-103.(=胶卷PB 85172)。 (2-Chloroethyl)-benzenesulfonate=“Benzolchlorethyl“ (Hoechst). 美国人译自德文。
反应式:本人有加注,译者未说明资料来源。
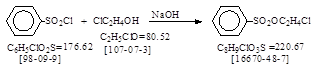
Equipment: 1. Homogenous lead-lined kettle with lead coil,
bottom outlet. Blade agitator 80 rpm –
cap. 2500 lit.
2. Measuring tank for
benzenesulfonyl chloride (BSC), steel homogenous lead-lined, with heating
coil. Cap. 1400 lit.
3. Agitated conical bottom
tank, wood cover, horizontal Hauer stirrer 50 rpm – cap. 5000 lit.
4. C.I. blowcase. 45 lbs.
working pressure 2000 lit. 5. Heated storage tank for BSC 1800 lit.
Procedure:
Into kettle (1) charge (by blowing with Nitrogen from pressure drums), 400 kg chlorohydrins and run from measuring
tank (2) 920 kg benzenesulfonyl chloride – under stirring and cooling (30 min.)
at such a rate that temperature does not exceed 25℃. (water cooling in winter
season, brine cooling during summer.).
Cool to 12-15℃. and shovel in – within 14-16 hrs. (using galvanized iron shovels) in 5 kg
portions 200 kg solid caustic soda. Temperature should not exceed 25℃. Before each addition cool to
12-15℃., after addition temperature increases thru
exothermic reaction. After completing
the caustic soda addition and stirring for 0.5 hr., reaction on Phenolphthalein
paper should be weak alkaline (pink), otherwise more caustic must be
added. Stir for 6 hrs. after the last
caustic addition, the alkaline reaction by this time should be constant. (Longer stirring causes loss yield, insufficient
stirring may leave unconverted BSC, which causes decomposition – with water –
in the subsequent distillation.). Add to
the thick paste in kettle (1) 1000 lit. water, stir for a few minutes then
drain contents, thru bottom outlet, into separation tank (3), which was
previously charged with 2000 lit. cold water.
Stir 10 minutes, then let it settle for 3 hrs. Separate – thru sight glass – the oil layer
into blowcase (4), water to sewer. Blow
back the oil layer into separator (3) which is charged with 3000 lit. cold
water, stir 10 minutes, settle for 3 hours and separate again. (Reaction must be alkaline on brilliant
yellow paper, if not, add 1-2 kg Soda).
The moist crude product (lower layer) is collected in drums for
distillation.
Distillation: Equipment:
Gasheated vacuum still, 1800
lit. capacity, blade agitator 30 rpm (1.5 KW) – equipped with fractionating
column 500 m/m Dia X 2800 m/m filled with Rashig rings to a height of 50 cm. One steel Liebig cooler. One coil cooler 40 m/m pipe Dia. One steel receiver 1500 lit. One steel receiver 500 lit.
Charge: 1125 kg crude moist
product. 5 kg calc. soda and for
subsequent batches. 40 kg first
fraction of the preceding batch.
Procedure:____________________________________________________________________________________________________________________
Time (hr)时间// Temp. ℃温度. of Still / Vapors //
Vacuum m/m. 真空度/ Still
/ Receiver // Fraction in kg. 产物。
2.5 – 3 //
165-175 to 125 // 16-12 /
10-8 //
1st fraction 40 kg
5 – 6 //
165-185 / 125-180 // 10 /
8 //
Main fraction 1000 kg.
190 /
150 // 10 / 8 // Last fraction 10 kg .
Residue 26 kg.
Yield: 1010 kg. Capacity: 2
distillations per day.
张澍声 《精细化工中间体工业生产技术》。 1996年。 P. 164-165. (2-氯乙基)苯磺酸酯。 译自FIAT 1313, I, 101.
在2500 L衬铅锅中用氮气压入400 kg 氯乙醇,再在搅拌和冷却下流入920 kg 苯磺酰氯,加入速度要使温度不超过25℃。冬天用冷水冷却,夏天用冰盐水冷却到12-15℃,于14-16小时内加入200 kg 固体氢氧化钠,每次加入5 kg,温度不超过25℃。每次加入前冷却到12-15℃,加料后由于放热反应温度上升。氢氧化钠加完后再搅拌30分钟,对酚酞试纸应呈弱碱性,否则必须再加氢氧化钠。最后一次加氢氧化钠,搅拌6小时,这时应始终保持碱性(较长时间搅拌引起收率降低;搅拌不充分会留下未反应的苯磺酰氯,在随后的蒸馏中会被水分解)。
向稠厚的膏状物中加入1000 L水,搅拌几分钟,由底部出口放入分液槽中,槽中预先加入2000 L冷水。搅拌10分钟,静置3小时,分出油层,油层回到分液槽中,加入3000 L冷水,搅拌10分钟,再分出油层(必须对亮黄试纸保持碱性,否则加1-2 kg 碳酸钠)。得到湿的粗产品。进行精馏。
在1800 L精馏锅(直径500 mm,高2800 mm,拉希环填充)中,加入1125 kg 湿的粗产品,5 kg 碳酸钠和上批精馏的40 kg 初馏份,进行精馏。(蒸馏表,见英文部分。)
德文原件:以下按美国人提供的胶卷目录,摘录如下:
PB 25602, 47-50. No. 11,626.“Benzolchloräthyl”(Benzenesulfonic
acid-β-chloroethyl ester). 德文生产工艺,未抄录。
PB 32533. IIE. No.6 Benzolchloräthyl
(Benzolsulfosäure-chloräthylester). 德文生产工艺,未抄录。
PB 70063, 551-555. Manufacture
of benzenesulfonic acid-β-chlorethyl ester. By weise. 1945年10月10日德文生产工艺,未抄录。
国内化工产品手册:
本人手头资料有限,未见有报导。
陈忠源 2019年8月19日星期一。













