CAS号 [6358-64-1] 生产工艺 4-氯-2,5-二甲氧基苯胺
CAS名: Benzenamine, 4-chloro-2,5-dimethoxy-, 参考文献: Beil. 13. E4, 2556.
用途: C.I. 冰染重氮组分23;44;112.
C.I. 颜料黄83.
C.I. 颜料红146.
生产工艺文献:
BIOS 986, 79-80. (=胶卷PB 77764) 4-Chloro-2,5-dimethoxyaniline (Crude) I.G. Griesheim. 英国人译自德文。抄录如下。
反应式: 本人有加注。译者未说明译自哪个PB报告,其实是译自德文PB 25625.
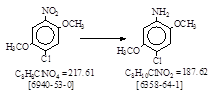
Iron reduction of
nitro body (No. 58。 见 BIOS 986, 105-107)。 Iron
reduction type 3 in presence of solvent.
2400 kg. Naphtha and 250
kg. 10% salt solution are charged to the reducer and boiled for 2 hours with 1500 kg. iron borings to obtain the iron in the required state of
acitivity. The mixture is kept at the boil
by the slow addition of 800 kg.
4-chloro-1-nitro-2,5-dimethoxybenzene 100% as wet filter cake. When reduction is complete the naphtha solution is drawn off from the
iron sludge which is then extracted with a further 800 l. naphtha.
The naphtha extracts are combined and filtered, the
naphtha steam distilled off and the amino
body granulated by cooling with agitation, and filtered cold. The iron
sludge can be left in the reducer for the next batch. The amount of fresh iron
borings being reduced to: 1000 kg. for the second batch and 500 kg. for the third and fourth
batches.
The reducer is cleaned out and after the fourth batch.
(Test for completion of reduction: after boiling has
subsided and the reaction mixture has been agitated for some time. 250 c.c. of naphtha solution from the
reducer is filtered hot to remove iron sludge, the naphtha is distilled off
with steam and the amino body granulated by cooling and filtered off. The amino
body is then dissolved in hydrochloric acid and should yield a clear solution:
if nitro body is still present the solution will be cloudy with nitro body in
suspension. In this case boiling and agitation are continued until reduction is
complete).
Materials consumption / tone of
4-chloro-2,5-dimethoxyaniline crude:
4-chloro-2,5-dimethoxynitrobenzene 1.23
t. Yield = 94% theory. Iron (compaign average )
1.6
t. Salt 0.06 t. Naphtha (gross) 4.0
t. (recovery not given)
Service consumptions / tone of 4-chloro-2,5-dimethoxyaniline
crude:
Electricity 824
K.W.H. Steam 19.2
t.L.P. Water 125 M3. Air 635 M3.
Analytical data: 4-chloro-2,5-dimethoxynitrobenzene M. Pt. 144-1450C. 4-chloro-2,5-dimethoxyaniline
crude C. Pt 1160C.
BIOS 986, 80-81. (=胶卷PB 77764) 4-Chloro-2,5-dimethoxyaniline puried. I.G. Griesheim. 英国人译自德文。抄录如下。
650 kg. crude 4-chloro-2,5-dimethoxyaniline
100% is added to 4000 l. water and 400 kg. hydrochloric acid 240Be’
at 50-600C. Agitation is
continued until the amino body is in solution. 50 kg. Carboraffin and approx. 60
kg. 40% bisulphate solution are then added and the batch filtered via a
wooden press to a vat. The free base is then precipitated at 400C. by the addition of
caustic soda liquor. The batch is then cooled and the base filtered off on a
vacuum filter and washed with water till neutral. The filter cake is them
centrifuged to reduce the water content and dried in vacuum.
Tests: (1) After addition of all the crude amino
body the mixture should be strongly acid to Congo Red.
(2) Before filtering through the press a
filtered sample should be only faint pink in colour. If it is darker more
carboraffin or bisulphite must be added.
Materials consumptions / tone of
4-chloro-2,5-dimethoxyaniline purified:
4-chloro-2,5-dimethoxyaniline crude 1.05
t. Yield =89.8% theory. (overall
from 4-chloro-1-nitro-2,5-dimethoxybenzene)
Hydrochloric acid 240Be’ 0.65
t. Caustic soda liquor 500Be’
as 100% 0.3 t. Carboraffin 0.08
t.
Services consumptions / tone of
4-chloro-2,5-dimethoxyaniline purified:
Electricity 130 K.W.H. Steam 0.6 t.L.P. Water 110 M3. Air 240 M3
Analytical data:
4-Chloro-2,5-dimethoxyaniline crude C.P. 1160C.
4-Chloro-2,5-dimethoxyaniline purified C.P. 1160C. the product to
be as light coloured as possible and solution in hydrochloric acid
red, not violet.
Annual production: 1937年 15000 kg. 1943年 6500 kg.
中文摘译文。张澍声 《精细化工中间体工业生产技术》1996年。 P. 90.(译自BIOS)请见原书。
译者不知道它译自德文生产工艺。
PB 25623, 598-603. 4-Chlor-2,5-dimethoxyanilin. 德文生产工艺规程,原件。未抄录。
BIOS 986. 的英文译文,是根据本德文生产工艺译出的。可惜本人未抄录全文,因此不能进行对比并加以说明。
国内研究动态:(部分摘录)
秦 龙 尹明娟 等。(辽宁大学) 硒催化CO/H2O体系高压还原制备2,5-二甲氧基-4-氯苯胺 [J] 染料工业,2016,3, 24-26(5)
1.2 实验步骤:
将一定量的2,5-二甲氧基-4-氯硝基苯,助催化剂,硒粉,环己烷,水加到100 ml 的高压反应釜内,高压釜密封后,将实验
所需量的CO充入,检测高压釜的气密性,再将高压釜置于油浴锅,设定反应温度及搅拌速度。当反应结束时,停止加热,将反
应釜从加热套中拿出,放到空气中自然冷却,将产物暴露在空气中搅拌,氧化一段时间后抽滤,并用蒸馏水洗涤2到3次。抽
滤后得到目标产物,避光烘干,称其质量,并用液相色谱检测产品的纯度。
4 结论:
最佳反应条件:硝基化合物:2.18
g (10 mmol), Se 0.0158 g (0.2 mmol), 助催化剂NaHCO3 0.42 g (5
mmol), 环己烷40
ml, H2O
2 ml; 反应温度1500C;
P co 6.0 MPa; 反应时间:8
h. 制备得到的2,5-二甲氧基-4-氯苯胺纯度可达98.38%,收率82.89%。
引用文献:
共8篇,其中含金属还原,加氢还原,水合肼还原和硫化碱还原,但未提到上述历史资料。至于硫氢化钠还原可参见CAS
号[97-50-7]生产工艺的历史资料。期刊引用文献。略!
加注:
上述抄录文献,是要说明生产工艺文献的收录,对了解产品的生产历史,对产品工艺创新还是有一定
意义的。问题是国内目前缺乏产品的系统索引?感谢作者的创新研究。
为什么资料没有引用历史文献?本人编写化学文库就是希望读者能利用这类历史文献。
陈忠源 2016年11月14日 于 无锡 明辉国际。













