CAS号 [101-57-5] 生产工艺 二苯胺-4-磺酸
CAS名: Benzenesulfonic acid, 4-(phenylamino)- 历史参考文献: Beil. 14. 699; E1, 721; E3, 2025; E4, 2691.
CAS名: Benzenesulfonic acid, 4,4’-iminobis- 历史参考文献: Beil. 14. 707. CAS号[112727-79-4]
用途: 酸性蓝93. 有机合成等。反应类别: 磺化。磺基水解。
生产工艺参考文献: 按本人手头资料整理如下。
BIOS 986, 187-190. (=胶卷PB 77764)。No. 119. Diphenylamine-4-sulphonic acid. (I.G. Mainkur). 英国人译自德文。
反应式: 本人有加注,译者未说明资料来源,本人暂未找到德文原件。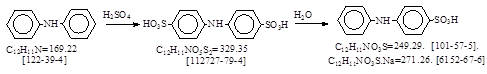
Materials charge per batch: Services consumptions: Manufacturing Expenses: Plant Description: 因有页面,今抄录部分:
(a) Disulphonation
of Diphenylamine:
500 l. of sulphuric acid 660Be’ = 920 kg. is measured
into Item 2 and 200 l. of this charge is run to the sulphonator. Item 20. To this charged, with agitation, 170 kg.
sodium sulphate and then 190 kg. diphenylamine during 1/2 hour. The temperature rises at first to 50℃. and then to 80℃. at which temperature solution takes place. Now heat to 128-132℃. during one hour and hold at this
temperature for 2 hours.
At 128-132℃. run in the remaining 300 l. of sulphuric acid 660Be’.
From Item 2 during 5-6 hours and hold at this temperature until a sample has
become clearly water soluble. This takes
12-15 hours.
Test 1: 5 c.c. of the sulphonation mass is poured into
95 c.c. of water and shaken well. The
solution must be bright yellow and quite clear.
If heating is continued any longer the solution become brown although
still quite clear but the lime out mass is very difficult to filter and the
yield deteriorates.
Higher temperatures and higher concentrations of
sulphuric acid produce bye-products.
(b) Splitting
to the monosulphonic acid:
The pan contents are cooled to 90℃. and blown to the split kettle, Item 3,
where the batch is cooled further to 70℃. From the
measure vessel, Item 4, is added 600 l.. of cold water during 5-6 hours.
Test 2: 49 g. of the diluted sulphonation is dissolved
in water and the volume adjusted to one litre. 100 c.c. is titrated with N/1 caustic
soda solution to phenolphthalein. If the
amount of water is sufficient the 100 c.c. require 40-41 c.c. N/1 caustic soda
solution. For each further c.c. a
further 10 l. of water is added.
The diluted mixture is heated to122-125℃. and held at this temperature for 3-4
hours until the completion of spilitting.
Test 3: 49 g. of the batch is diluted with 50 c.c. of
water and made weakly alkaline with about 50 c.c. caustic soda solution 330Be’. The colour changes from bright brown to
bright green. This solution is poured
hot into 400 c.c. of boiling 25% barium chloride solution. The precipitate (diphenylamine monosulphonic
acid barium salt and barium sulphate) is cooled to 20℃. filtered and washed with 25% barium
chloride solution until the volume is 500 c.c.
This filtrate is titrated at 10℃. with N/1 sodium nitrite solution. 不再抄了!
细田豊 《理论制造染料化学》 (日)技報當 出版。 1957年。P. 470. 28. ジフェニルアミン-4スルホン酸[2]
译自PB 77764. 无页号!本文为摘译文。
张澍声 编译 《精细化工中间体工业生产技术 – BIOS FIAT
PB Reporter》《染料工业》编辑部 出版。 1996年。P. 8-9.
018 二苯胺-4-磺酸。 译自BIOS 986, 187. 本译文比日本人详细
抄注: 老产品新用途,这应该是创新吧![112727-79-4]主要是用于铝表面的着色助剂。如感兴趣,请査CA.
陈忠源 2017年12月14日。













