CAS号
[94-45-1] 生产工艺 2-氨基-6-乙氧基苯并噻唑
CAS名:
2-Benzothiazolamine, 6-ethoxy- 历史参考文献:
Beil. 27. E2, 335.
用途: 冰染偶合组分9。 碱性蓝66,
67。 分散红64。 反应类别: 硫脲化,闭环。
生产工艺参考文献: 本人按手头资料整理如下。
BIOS
1149, 82-83.(=胶卷PB
80376)2-Amino-6-ethoxybenzthiazole
(for
2-Acetoacetamido-6-ethoxybenzthiazole – Naphtol AS-L4G)
反应式: 本人有加注。本资料是英国人译自德文,生产厂(Mainkur),未说明资料来源。德文原件暂未找到!
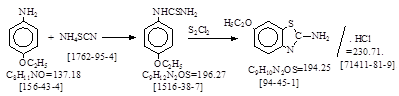
Materials: p-Phenetidine (M.W. 137) 205 kg.
Chlorobenzene 1000 l.. Monohydrate
75 kg. Sodium thiocyanate (anhyd.) 123 kg.
Sulphur
chloride 243 kg. Caustic liquor 650 l. + 275 l. Hydrochloric acid 225 l.
Procedure:
(a) Formation of thiourea: Into an enameled vessel is charged 1000 l. of
chlorobenzene and 205 kg. of p-phenetidine.
Then 75 kg. of monohydrate are added from the sulphate. The suspension is cooled to 350, 123 kg. of
sodium thiocyanate (anhyd.) added, heated at 900 for 4 hours, and then cooled.
(b)
Ring-closure: 243 kg. of sulphur
chloride are added, the temperature rising rapidly to 75℃. It is maintained 2
hours at 75-80℃., cooled to 300 and then run into 500
l. of water and 650 l. of caustic liquor.
The chlorobenzene is steam-distilled off, the product filtered and
washed.
(c)
Purification: The crude product is
dissolved in 3000 l. of water and 225 l. of hydrochloric acid at 50℃., screened, and the filtrate basified with 275 l. of caustic
liquor. The precipitated
6-ethoxy-2-amino-benzthiazole is filtered, washed and dried.
Yield
= 262 kg. act. wt. assumed 100% (M.W. 194) = 90.2% theory.
FIAT
1313,I, 33-36.(=胶卷PB
85172)
2-Amino-6-ethoxybenzthiazole. (I.G. Mainkur) “Amidothiaazol”. 美国人译自德文无资料来源
p-Phenetidinsulfate
in converted – with Rhodanammonium in Chlorbenzene suspension – into Rhodamide,
then thru heating rearranged to p-ethoxy-phenylthiourea and thru rapid addition
of sulfur chloride a labile intermediate condenses at increased temperatures to
Amidothiazol, under HCl evolution.
Charge:
Chlorbenzene 1650 kg. 1.5 moles p-Phenetidine 205.5 kg.
1.57 moles Rhodanammnium (5% excess)
123.0 kg. 0.75 moles Monohydrate 75.0 kg.
1.8 moles Sulfur chloride (20% excess)
243.0 kg. Caustic soda 330Be’-
925 liter 1200.0 kg. Muriate Acid
270.0 kg.
Yield: 1000 kg presscake containing 262 kg dry pure
= 90% of theory. Chlorbenzene losses 6%.
Procedure:
In
enameled 2000 l. kettle (1) charge 205 kg p-Phenetidine, 1050 lit Chlorbenzene
(dry), then run in while stirring well, thru drop funnel, in 1 hr. 75 kg
Monohydrate. Temperature not over 35℃. (Test 1). Add 123 kg
Rhodanammonium and heat to 90℃. To complete the thiourea formation keep 4
hours
at
this temperature. (Test 2). Cool charge
to 30℃. and blow over into enameled kettle
(2), rinsing kettle (1) with 250 l. chlorbenzene. Into this suspension
blow
in quickly (in 1-2 min.) 242 kg Sulfurchloride.. The reaction sets in very soon under
temperature rise (to 75℃.) and HCl
evolution, and the kettle must
be
closed quickly. Heat to 75-80℃. and keep temperature for 2 hours. (Test 3). Volume 1700 lit. Cool to 30℃.
and blow to 22000 lit. capacity still (3)
which
was charged previously with 650 lit Caustic soda 330Be’ and 500 lit Water. Chlorbenzol is distilled off with open steam
into receiver (4). Due to the
fact
that NH3 is distilled off simultaneously, the chlorbenzene must be acidified
with H2SO4 before drying, mixed well and separated. The residue of
distillation
is yellowish, finely grained and heavy like sand. Thru short pipe line blow to suction filter
and wash free of Na2S containing mother liquor with
approx.
500 l. water.
The
filter cake is charged into kettle (6) which is previously charged with 225 l.
muriatic acid and 3000 l. water at 50℃. After 3-4 hrs stirring the Amidothiazol goes
into solution and filtered and washed thru press (7) to remove the sulfur
(about 90 kg residue), (Test 4). The
acid filtrate is collected thru lead pipe line into kettle (8) which is charged
with 275 l. caustic soda and sufficient water to make stirring feasible.
The
Amidothiazol precipitates in voluminous form, almost pure white (Test 5), about
5000 lit. Filtered thru press (9) and
the kettle is used with small amount of cold water.
1000
kg paste is obtained, which is dried in vacuo at 50-60℃., or in air dryer at 70-750 (rust free trays). The pulverized and mixed Amidothiazol is
shipped to Hoechst where it is condensed with acetic acid ester to Naphthol
ASL4G.
Tests: Rhodanammonium is very hygroscopic and should
not be kept in iron drums. Chlorbenzene
must be waterfree.
1. Sulfate test: All phenetidin present
should be as Sulfate insoluble in chlorbenzene.
Chlorbenzne filtrate treated with HCl and nitrit should not couple with
R Salt. There should be no excess H2SO4. Sample diluted with water should not be acid
reactive to methyl orange.
2.
Thiourea is filtered and resludged in water, test for excess of Rhodanammonium
with FeCl3 solution. Red brown. Washed and dried sample should have M.P.
170-172℃.
Yield: 97% of theory. Chlorbenzol
filtrate, distilled alkaline with steam, should show almost no precipitate when
acidified, and should contain only approx. 1 1/2 % (of charge) diazotization.
3.
Sample filtered should give granular cake and water clear filtrate. Gelatinous filtrate indicates insufficiency
of S2Cl2 , which cannot be corrected with subsequent additions.
4.
The residue shall be free of Aminothiaole.
Sample rolled with dil. HCl for 2 hrs. filtered, filtrate neutralized
with caustic should show no precipitate.
5.
M.P. (156- ) 161-163℃. Purity can
be determined thru alcohol extraction.
Sulfate ash content (in residual soda) 6-8%.
Equipment: 共11台,不再抄录。
2-Amino-6-ethoxybenzthiazole,
pure. (I.G. Mainkur – 1937年1月4日) 美国人译自德文。
Charge
the solution kettle with500 k. 2-amino-6-ethoxybenthiazole crude 100%, about
1500 – 1800 k. paste, dilute to 2000 l. add 300 l. hydrochloric acid to a good
Congo acidity. Heat to 80℃. to dissolve. Add 30 k.
Decolorizing carbon, filter, add to the filtrate , 600 l. hydrochloric acid,
cool to 20℃. filter, wash with 500 l. Salt
solution. Dissolve the paste in 1500 l.
water at 70℃. and add 10 k. Decolorizing
carbon. Filter into 350 l. sodium
hydroxide solution, 330Be’, and enough water to agitate. Filter and wash as free of alkali as
possible. Dry at 70-75℃. in air. Grind and mix.
The
Yield is 80% of theory. The product has
M.P. 161-163℃.
Iron content less than 0.2%.
The
form of the hydrochloride salt (抄注:CAS号[71411-81-9])varies,
some charges are thin, some are so thick that they are difficult to
agitate. The latter generally have to be
clarified again.
请读者注意: 同一德文生产工艺,美国人的译文就比英国人详细,其实他们能提供不懂德文的读者参考已不容易了。
PB
70061, 1582. 6-Aethoxy-2-aminobenzthiazol. 产品标准。
这里说明有德文生产工艺原件,只是本人未找到。
PB
70190, 7446-7449.
6-Aethoxy-2-aminobenzthiazol. Nr. 549.
1936年产品分析方法。
张澍声
《精细化工中间体工业生产技术》
1996年, p.
261-262. 2-氨基-6-乙氧基苯并噻唑。
摘译自FIAT 1313, I, 33; BIOS 1149, 82。
本人不再抄录,请读者谅解。
随笔:
记得大学毕业后参加研究工作,要找一篇参考文献有多难,后来发现Lubs的合成染料和颜料化学和细田豊的理论制造染料化学(也就是沈阳化工研究院才有可能进口的图书),找到了一条加快研究进度,开发思路的道路。研究创新是我们努力的方向,我想总应该有一些基础资料吧!
在工厂第一线工作的技术人员,要找一篇参考文献应该更难,这就是本人建立网页的初衷,问题是本人已老了,总有一天会离开大家,如果我的想法是对的,那么接班人在哪里?英文是Succesor,俄文有过”Cмена”接班人的杂志,当然不能自以为了不起,没有我地球照样转。
一个人能力有大有小,我想本人还是有点小吧。不是Zero! 现在流行正能量,也应该不是负能量吧!
一分耕耘一分收获。The more ploughing and weeding, the better the crop. 这里的收获是对读者有用,不是回报!希望如此。
一位不要任何回报的单干户。如果还有点用,希望有Смена!本人至少近期还有能力共同讨论,共同进步。
现在是想报名参考志愿者团队,也不知道到哪里去报名!只有继续单干!
陈忠源 2018年1月28日













