CAS号 [83732-61-0] 生产工艺。 6-氯-2-硝基苯酚-4-磺酸
CAS名:Benzenesulfonic acid, 3-chloro-4-hydroxy-5-nitro- Beil.
11, 247.
用途:有机合成,染料中间体。
LookChem网登录3家。 反应类别:硝化。
BIOS 1153, 222-223.(=胶卷PB 85687)。 6-Chloro-2-nitrophenol-4-sulphonic acid. (Leverkusen). 英国人译自德文,无资料来源。
反应式:暂未找到德文原件。
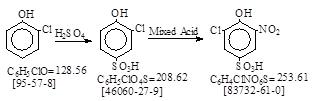
Outline: 概要:
o-Chlorophenol is sulfonated and then nitrated; the resulting isolated
6-chloro-2-nitrophenol-4-sulfonic acid.
Plant Details: 设备:
1 3000 l. cast iron kettle with
steam and cooling jacket. 1 10000 l. vat with brine coils. 1
Nutsche.
Materials: 投料量:
300 kg. o-chlorophenol (pure) ≈ 160 kg. NaNO2.
1800 kg. H2SO4.
372 kg. Mixed Acid A(40% HNO3 + 48% H2SO4 + 12% H2O)≈149 kg. 100% HNO3 = 102% theory.
Process: 操作步骤:
Sulphonation: 磺化: 300 kg. of o-chlorophenol are charged into the 3000 l. sulphonation
vessel containing 600 kg. of 98% H2SO4.
The temperature is kept around 60 – 70℃. and, when all is in, is raised to 95 – 100℃. for 6 hours. A drop should
provide a clear solution in water.
Nitration: 硝化:The sulphonation mixture is now diluted with 1200 kg. of 98% H2SO4 and
150 kg. of water. It is cooled to 20℃. and at 20 – 25℃. (not lower as the mass becomes too thick)
nitrated with 372 kg. of mixed acid A.
After the final addition it is tirred 2 hours longer. The
finished nitration is blown into 4000 l. of water and cooled by means of the
brine coils to 15 – 20℃. A
trace of the dinitro derivative (about 1% is removed by filtration.
The liquors are then salted out (test for completion of precipitation)
and re-filtered. Yield : 75% theory.
张澍声《精细化工中间体工业生产技术》。《染料工业》编辑部 出版。1996年。P. 82.
6-氯-2-硝基苯酚-4-磺酸,译自BIOS 1152.
(1)磺化:在3000 L铸铁磺化锅中加入600 kg 98% 硫酸,再加入300 kg 邻氯苯酚,相当于160 kg 亚钠量,温度保持在60 – 70℃。当全部加完后,升温至95 – 100℃。保持6小时。一滴样品加到水中,应为清晰溶液。
(2)硝化:在上述磺化混合物用1200 kg 98% 硫酸和150 kg水稀释,冷却到20℃,在20 – 25℃(不要太低使物质变得太粘稠)用372 kg 混酸(48% 硫酸,40% 硝酸,12% 水)硝化。加完混酸后再搅拌2小时。
硝化后的物料压入4000 L水中,用冰盐水冷却至15 – 20℃。过滤除去约1% 的二硝化衍生物。
溶液再盐析并过滤,盐析要检验沉淀是否完全。收率75%。
何岩彬 主编《染料品种大全》。沈阳出版社 出版。2018年。P. 1864. 中文名称:3-氨基-5-氯-4-羟基苯磺酸。
CAS:5857-94-3. 【可合成的染料】C.I. 酸性蓝36;C.I. 直接红259;C.I. 直接红261;C.I. 直接紫53;C.I. 活性紫1。已在9月26日上网。
【抄注】它是由本化合物还原合成,所以本化合物应列入p. 1859页上,其【可合成的染料】同上。
陈忠源 2020年9月28日星期一。













