CAS号 [50-84-0] 生产工艺。 2,4-二氯苯甲酸
CAS名:Benzoic acid, 2,4-dichloro- 历史参考文献:Beil. 9, 342; E1, 141; E2, 228; E3, 1374; E4,
998.
用途:橡胶助剂中间体,医药中间体等。LookChem网登录生产与经营单位114家。
BIOS 986, 152-154.(=胶卷PB 77764)。 2,4-Dichlorobenzoic acid (I.G. Ludwigshafen). 英国人译自德文(无资料来源)。
反应式:本人有加注,有两条工艺路线。
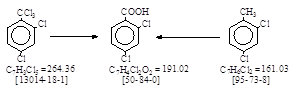
Process (A): The
following is the original Ludwigshafen Process given in a confidential document
date 18. 10. 1941 from Ludwigshafen to Hoechst following damage to the Ludwigshafen
plant. Plant: 略。
Process: 操作步骤:(译自德文,其中有两位译者,指BIOS 986 和BIOS 1145.)。
Charge 3,000
kg. sulphuric acid 600Be’ to the pan heat to 85-90℃. In
the course of 30-36 hr. run in slowly and steadily 2,000 kg,
2,4-dichlorobenzotrichloride. The
evolved hydrochloric acid is lead off.
Stir for 6 hours at the same temperature after the addition is
complete. Test a sample in the
laboratory to see that the hydrolysis is quite complete. With stirring add about 4,000-6,000 kg. water
whereby the dichlorobenzoic acid is precipitated as a sandy heavy mass. Filter on the nutsche, suck dry and
wash. Dissolve in the wooden vat with an
excess of a 10% soda solution and evaporate in the steam distillation
apparatus. If a trial precipitation
shows that the acid is not pure white, purity by the addition of a little
hypochlorite, and then screen again through the press into the wooden vat which
has been cleaned in the meantime. The
clear filtrate is diluted with 10-12% of water and precipitated at 80℃. with dilute sulphuric acid. Cool, centrifuge and wash neutral.
Yield: 1,380
kg. 95.7%.
Note: The
ortho chlorine atom in 2,4-dichlorobenzoic acid is somewhat labile and can be
replace by –OH. On these groups copper, tin and zinc must be rigeoously
excluded from the hydrolysis vessel. By
following this advice the p-chlorosalicylic acid content can be kept down to
traces, say 0.1-0.5%. The maximum
permissible is 1.0%. It is suggested
that traces of Ni might promote a similar reaction.
Process (B): This
is the Hoechst process based on five batches made in November-December 1941 in
F. 50.
Charge 1,750
l. = 3,000 kg. sulphuric acid 600Be’. 78% so that 1-2 turns of the coil in the
hydrolysis vessel are covered. The
charge-hole is closed with a wooden cover leaving only a small opening. Then during the course of 20-24 hrs. shovel
in about 1,400 kg. dichlorobenzotrichloride.
This was delivered in the form of lumps in casks from the Methyl
Department and had M. Pt. 42-43℃. The material was quite
colourless and contained only a little oil (phosphorus oxy chloride). The lumps were broken up in the diphenylamine
crusher and charged to the sulphuric acid at 80-90℃. The
hydrochloric acid was absorbed in water in a spray tower, the dilute
hydrochloric acid being run to drain. No
trouble of any sort was experienced with the hydrochloric acid. Maintain at 80-90℃. by occasional gentle heating. When about 2/3 of the trichloride has been
added a slurry is formed, but it remains easily stirrable. Hold at 90℃. for 24 hours. The whole process takes about 40-48 hours to
this stage.
Cool with
water, and run on to about 1,000 kg. ice with the simultaneous addition of
water to the top of the vessel. Cool
with the coil to 30-35℃. and filter on a nutsche. Wash
acid-free with 25 cu. m. water. Divide
the nutsche contents into two parts.
Dissolve with 4,000 l. water and about 100 kg. soda solution at 80-90℃. to give faint alkalinity. The only faintly soloured bright solution is
clarified with 10-20 kg. Esbithocarbon (vol. 5,000 l.), heated to 80℃ and 200 l. hydrochloric acid added to
distinct Congo Red acidity. Cool to 40℃. and filter.
Stir up with water in the precipitation vessel at 35℃., filter again, and wash with 7.5 cu.
m. The paste is now neutral. Dry off on the nutsche to 30-35% solids and
centrifuge to 55% solids. Dry in the
vacuum dryer at about 500 mm. vacuum.
The inside of the dryer becomes covered with sublimed product. Dry for 24-36 hours and grind. A 150 l. cask holds about 80 kg. Strength: 98.25 – 98.5% dichlorobenzoic
acid. About 0.5% p-chlorosalicylic acid.
Yield:
780-850 kg. = 76.83% theory.
张澍声 《精细化工中间体工业生产技术》 1996年。 P. 138-139. 译自BIOS 986, 152. 2,4-二氯苯甲酸。
下面是Ludwigshafen厂 1941年10月18日的保密生产工艺A,二次世界大战被毁后有了Hoechst厂的生产工艺B。
(一)工艺A:在一均匀衬铅的搅拌锅中加入3000 kg 78% 硫酸,加热到85-900。 于30-36小时内缓慢而稳定地流入2000 kg 2,4-二氯三氯甲苯,导出释放的氯化氢。加料完毕后在同样温度下搅拌6小时,取样检验水解是否十分完全。在搅拌下加入4000-6000 kg 水,二氯苯甲酸以沙粒状沉淀出来。真空抽滤并洗涤。在木槽中用过量10% 的碳酸钠溶解滤饼,并在水蒸汽蒸馏设备中蒸发。如果试样沉淀不是纯白色,加入少量次氯酸钠精制,然后压滤到洗净的木槽中。清洁的滤液用10-12% 水稀释,在80℃用稀硫酸沉淀,冷却,离心,水洗至中性,得1380 kg产品,收率95.7%。
注意:2,4-二氯苯甲酸的邻位氯原子较为活泼,能被OH基所取代,因此在水解容器中必须完全排除铜,锡和锌。遵守这一忠告,对氯水杨酸含量能够降至痕量,0.1-0.5%,最大允许量为1.0%。痕量的镍也能促进类似的反应。
(二)工艺B:水解器中加入1750 L 78% 硫酸(3000 kg),使水解器的1-2圈蛇管被遮盖,用木盖关闭加料孔,仅留一小开口。然后于20-24小时内加入约1400 kg 二氯三氯甲苯,二氯三氯甲苯为完全无色的块状,熔点42-43℃,仅含少量的油(磷酰氯),先将块状物在压碎机中粉碎,在80-90℃加到硫酸中,生成的盐酸在喷雾塔中吸收在水中,稀盐酸排放出去。维持80-90℃有时要温和地加热。当约2/3的二氯三氯甲苯加入后,形成浆状物,但它仍然是容易搅拌的。在90℃保持24小时。整个工艺约需40-48小时。
用水冷却,加入1000 kg冰,同时由容器顶部加水,蛇管冷却到30-35℃,真空过滤,用25000 L水洗至不含酸。将滤饼分为两份。在80-90℃用4000 L水和 约100 kg碳酸钠溶液溶解,为弱碱性。用10-20kg活性炭将略带浅色的5000 L溶液脱色,加热至80℃,加入200 L盐酸至对刚果红为明显酸性。冷却到40℃并过滤,滤饼在35℃于沉淀容器中与水一起搅拌,再过滤,用7500 L水洗涤,滤饼为中性,抽干至含固量30-35%,离心至含固量55%,在真空干燥器中于约500 mmHg干燥。干燥器内壁被升华产品所覆盖,干燥24-36小时,并粉碎。
得到780-850 kg
98.25-98.5% 的2,4-二氯苯甲酸,约含0.5% 对氯水杨酸。收率:76.83%。
BIOS 1145.(=胶卷PB 80345)。Manufacture of chlorotoluene and derived
nitro chlorotoluenes. 英国人的译文本。本人未收藏。
张澍声 《精细化工中间体工业生产技术》1996年。 P. 122. 2,4-二氯苯甲酸。 译自BIOS 1145, 36.
在7 m3 水解锅中先加入3000 kg 78% 硫酸,再于24小时内流入1725 kg 2,4-二氯三氯甲苯,加料是于搅拌下在85-90℃进行。在此温度再加热6小时,检验水解的完成,取样水洗后溶解于碳酸钠,应无油珠分出。反应物冷却到50℃,搅拌加入足够的水,使温度升至60-70℃。冷却到40℃后抽滤,很好洗涤。
在18 m3 木槽中加入8000 L水,加热到80℃,在热水中溶解1000 kg 碳酸钠。将上述粗品2,4-二氯苯甲酸加入,搅拌至溶解,过滤。滤液再加热至80℃,用70 kg次氯酸钠溶液处理,用78% 硫酸沉淀出游离酸,搅拌冷却到40℃,产品离心过滤,洗涤并干燥。收率97-99.5%。
2,4-二氯苯甲酸含量99.5%(下限98.5%),氯代水杨酸0.02% (上限1.0%),熔点161-163℃。
PB 25602, 1280. 2,4-Dichlorobenzoic acid. 德文生产工艺原件。 本人未抄录。
PB 70190, 7982. 2,4-Dichlorobenzoic acid. 德文产品分析方法。 本人未抄录。
国内出版物:
徐克勋 主编 《有机化工原料及中间体便览》 1978年。 P. 484.
2,4-二氯苯甲酸。(无资料来源)。
2,4-二氯甲苯用5% 氢氧化钠洗,再用水洗,用高锰酸钾进行氧化,然后过滤,除去二氧化锰,用盐酸酸化,水结晶而得成品。
侯乐山 主编 《中国精细化工产品集 – 原料及中间体10396种》 2006年。 P. 411-412.
2,4-二氯苯甲酸。
中国化工信息中心 全国精细化工原料及中间体行业协作组 出版 (版权所有 未经允许 不得翻印)
生产方法:本人核对,它与徐克勋的资料完全相同。
张林栋 编 《化工产品手册,第五版 – 橡塑助剂》 2008年。 P. 375. 硫化剂DCBP。 2,4-二氯苯甲酸的制备。
将吡啶,2,4-二氯甲苯及高锰酸钾依次加入水中,搅拌下升温至70℃,保持此温度搅拌反应4 h。磺酸吡啶,反应系统蒸干后再加水溶解。过滤,滤液用酸调节至pH值等于2,析出沉淀,再经过滤,得2,4-二氯苯甲酸。 (抄注:无资料来源。)。
请读者评述。
陈忠源 2018年9月22日星期六。













